- Task
- Practice Exercises
1. The molecular mass of a substance#
Notes:
The molecular mass of a substance is the sum of the average masses of the atoms in one molecule of a substance. It is calculated by adding together the atomic masses of the elements in the substance, each multiplied by its subscript (written or implied) in the molecular formula. Because the units of atomic mass are atomic mass units, the units of molecular mass are also atomic mass units. The procedure for calculating molecular masses is illustrated in Example 1.
Example#
Calculate the molecular mass of propanol, whose condensed structural formula is
CH3CH2CH2OH
Given: molecule
Asked for: molecular mass
Strategy:
A. Determine the number of atoms of each element in the molecule.
B. Obtain the atomic masses of each element from the periodic table and multiply the atomic mass of each element by the number of atoms of that element.
C. Add together the masses to give the molecular mass.
Solution:
A. The molecular formula of propanol may be written in three different ways: CH3CH2 CH2OH (which illustrates the presence of a propyl group, CH3CH2CH2−, and an −OH group), C3H7OH, and C3H8O; all show that propanol has three carbon atoms, eight hydrogen atoms, and one oxygen atom.
B. Taking the atomic masses from the periodic table, we obtain:
3×atomic; mass of carbon=3 atoms · (12.011amu/atom) =36.033amu
8×atomic; mass of hydrogen=8 atoms · (1.0079amu/atom) =8.0632amu
1×atomic; mass of oxygen=1atoms · (15.9994amu/atom) =15.9994amu
C. Adding together the masses gives the molecular mass:
36.033amu + 8.0632amu + 15.9994amu = 60.0956amu
Alternatively, we could have used unit conversions to reach the result in one step:Mr(C3H8O) = [3 atoms · (12.011amu/1atom C)] + [8 atoms H · (1.0079amu/1atom H)] + [1atoms O · (15.9994amu/1atom O)]
Answer:
the molecular mass of propanol is 60.0956amu or Mr(C3H8O) =60.0956amu
Сheck yourself:
Calculate the molecular mass or formula mass of each compound:
Sulfuric acid
Calcium nitrate
Ammonium phosphate
Explain a solution strategy using key expressions
2. Molar Mass#
Notes:
The molar mass is defined as the mass in grams of 1 mol of that substance. One mole of isotopically pure carbon-12 has a mass of 12 g. For an element, the molar mass is the mass of 1 mol of atoms of that element; for a covalent molecular compound, it is the mass of 1 mol of molecules of that compound; for an ionic compound, it is the mass of 1 mol of formula units. That is, the molar mass of a substance is the mass (in grams per mole) of 6.022 × 1023 atoms, molecules, or formula units of that substance. In each case, the number of grams in 1 mol is the same as the number of atomic mass units that describe the atomic mass, the molecular mass, or the formula mass, respectively. The molar mass of any substance is its atomic mass, molecular mass, or formula mass in grams per mole. The periodic table lists the atomic mass of carbon as 12.011 amu; the average molar mass of carbon—the mass of 6.022 × 1023 carbon atoms—is therefore 12.011 g/mol
| Substance (formula) | Atomic, Molecular, or Formula Mass (amu) | Molar Mass (g/mol) |
|---|---|---|
| silicon (Si) | 12.011 (atomic mass) | 12.011 |
| propanol (C3H7OH) | 60.0956amu (molecular mass) | 60.0956 |
| Sodium carbonate [Na2CO3] | 105.98874amu (formula mass) | 105.98874 |
Сheck yourself:
1.What is the total number of atoms in each sample?
a) 0.545 mol of NaOH
b) 1.42 mol of CO2
2. Calculate the mass in grams of each sample.
a) 2.56 mol of CaCO3
b) 0.45 mol of SO3
3. Calculate the number of moles in grams of each substance.
a) 5.6g of HNO3
b) 1.8g of Na3PO4
Explain a solution strategy using key expressions
3. The Mole#
Notes:
Units of:
n – mole
m – g
M – gmole-1
Example 1.#
Calculate the number of moles and molecules of oxygen in 4g oxygen molecules with the formula O2
Given: mass and molecular formula
Asked for: number of moles and number of molecules
Strategy:
A. Use the molecular formula of the compound to calculate its molecular mass in grams per mole.
B. Convert from mass to moles by dividing the mass given by the compound’s molar mass.
C. Convert from moles to molecules by multiplying the number of moles by Avogadro’s number.
Solution:
A. The molecular mass of oxygen can be calculated from its molecular formula using the method:
Mr(O2) = (2 atoms of O) · (15.9994amu/atom O) = 31.9988amu
The molar mass of oxygen is 31.9988 g/mol
B. The number of moles of oxygen present in 4g can be calculated by dividing the mass (in grams) by the molar mass (in grams per mole):
C. To calculate the number of molecules in the sample, we multiply the number of moles by Avogadro’s number:
Answer:
n(O2) = 0.125 mol; N(O2) = 0.7525 × 1023 molecules
Example 2.#
Calculate the mass of 1.75 mol of each compound:
- SO2 (sulfur dioxide)
- Na2CO3 (sodium carbonate)
Given: number of moles and molecular or empirical formula
Asked for: mass
Strategy:
A. Calculate the molecular mass of the compound in grams from its molecular formula (if covalent) or empirical formula (if ionic).
B. Convert from moles to mass by multiplying the moles of the compound given by its molar mass.
Solution:
We begin by calculating the molecular mass of SO2 and the formula mass of Na2CO3.
A. The molar mass of SO2 is obtained from its molecular mass as follows:
Mr(SO2) = (1 atom of S) · (32.065amu/atom S) + (2 atoms of O) · (15.9994amu/atom O) =32.065amu + 31.9988amu = 64.0638amu
The molar mass of SO2 is 64.0638 g/mol.
The mass of 1.75 mol of SO2 is calculated as follows:
m(SO2) = n · M
m(SO2) = 1.75 · 64.0638 gmol-1 = 112.1165g
B. The formula mass of Na2CO3 is obtained as follows:
The formula mass [Na2CO3] = [(2 atoms of Na) · (22.98977amu/atom Na) + (1 atom of C) · (12.011amu/atom C)+ (3 atoms of O) · (15.9994amu/atom C)] = 45.97954amu +12.011amu +47.9982amu =105.98874amu
The molar mass of Na2CO3 is 105.98874 g/mol.
The mass of 1.75 mol of Na2CO3 is calculated as follows:
m(Na2CO3) = n · M
m(Na2CO3) = 1.75 · 105.98874gmol-1 = 185.48029g
Answer: m(SO2) = 112.1165g; m(Na2CO3) = 185.48029g
Сheck yourself:
- Define «1 mol of carbon atoms»
- Describe the relationship between an atomic mass unit and a gram.
- Is it correct to say that propanol has a formula mass of 60? Why or why not?
- If 2 mol of calcium reacts completely with 1 mol of oxygen to produce calcium oxide, does this mean that 2 g of calcium reacts completely with 1 g of oxygen to give the same product? Explain your answer.
- Construct a flowchart to show how you would calculate the number of moles of:
A. phosphorus in a 60.0 g calcium phosphate (Ca3(PO4)2), used in the manufacture of mineral fertilizers
B. carbon in a 68.4 g sample of sucrose that contains 42.1% carbon by mass.Explain a solution strategy using key expressions
4. Calculating Mass Percentages#
Notes:
Mass percentage is calculated as the mass of a component divided by the total mass of the mixture, multiplied by 100%
The percent composition AxBy of a compound can also be determined from the formula of the compound. The subscripts in the formula are first used to calculate the mass of each element in one mole of the compound. That is divided by the molar mass of the compound and multiplied by 100%
Example#
Glycine is the simplest amino acid that reduces psycho-emotional stress and increases mental productivity. Its molecular formula is C2H5NO2. Calculate the mass percentage of each element in glycine. Calculate the mass of carbon in a 1.00 g packet of Equal, assuming it is pure glycine.
Given: molecular formula and mass of sample
Asked for: mass percentage of all elements and mass of one element in sample
Strategy:
A. Use atomic masses from the periodic table to calculate the molar mass of glycine.
B. Divide the mass of each element by the molar mass of glycine then multiply by 100 to obtain percentages.
C. To find the mass of an element contained in a given mass of glycine, multiply the mass of glycine by the mass percentage of that element, expressed as a decimal.
Solution:
A. We calculate the mass of each element in 1 mol of glycine and the molar mass of glycine, here to three decimal places:
| 2 C | (2 mol C)(12.011 g/mol C) = | 24.022 g |
| 5 H | (5 mol H)(1.008 g/mol H) = | 5.04 g |
| 1N | (1 mol N)(14.007 g/mol N) = | 14.007 g |
| 2O | (2 mol O)(15.999 g/mol O) = | 31.998 g |
| C2H5NO2 | molar mass of aspartame | 75.067 g/mol |
B. To calculate the mass percentage of each element, we divide the mass of each element in the compound by the molar mass of glycine and then multiply by 100 to obtain percentages, here reported to two decimal places:
As a check, we can add the percentages together: 32% + 6.714% + 18.659% + 42.627% = 100.00% If you obtain a total that differs from 100% by more than about ±1%, there must be an error somewhere in the calculation.
C. The mass of carbon in 1.00 g of glycine is calculated as follows:
Answer: m % C = 32%; m % H = 6.714%; m % N = 18.659%; m % O = 42.627%.mo f C in 1 g glicine = 0.2934 g
5. Determining the Empirical Formula#
Notes:
An empirical formula represents the lowest whole-number ratio of elements in a compound.
To find an empirical formula when given percent composition we need:
- To assume that you have 100 g of the unknown compound;
- To convert the masses from Step 1 into moles using the molar mass;
- To determine which element has the smallest mole value. Then divide all the mole values you calculated in Step 2 by this smallest value;
- If any of your mole ratios aren’t whole numbers, multiply all numbers by the smallest possible factor that produces whole-number mole ratios for all the elements;
- To write the empirical formula by attaching these whole-number mole ratios as subscripts to the chemical symbol of each element.
Example#
Calculate the empirical formula of the ionic compound calcium carbonate, used as building material. Elemental analysis indicates that it contains 40% calcium, 12% carbon, and 48% oxygen.
Given: percent composition
Asked for: empirical formula
Strategy:
A. Assume a 100 g sample and calculate the number of moles of each element in that sample.
B. Obtain the relative numbers of atoms of each element in the compound by dividing the number of moles of each element in the 100 g sample by the number of moles of the element present in the smallest amount.
C. If the ratios are not integers, multiply all subscripts by the same number to give integral values.
D. Because this is an ionic compound, identify the anion and cation and write the formula so that the charges are balanced.
Solution:
A. A 100 g sample of calcium carbonate contains 40 g of calcium, 12 g of carbon, and 48 g of oxygen. Dividing the mass of each element in the 100 g sample by its molar mass gives the number of moles of each element in the sample:
B. To obtain the relative numbers of atoms of each element in the compound, divide the number of moles of each element in the 100-g sample by the number of moles of the element in the smallest amount, in this case calcium:
C. We can write the empirical formula of calcium carbonate as Ca1.000С1.001O3.007. The deviation from integral atomic ratios is small and can be attributed to minor experimental errors; therefore, the empirical formula is CaCO3.
D. The calcium ion (Ca2+) is a cation, carbonate ion (CO32-) is an anion. Hence the formula of the ionic compound is CaCO3
Answer: CaCO3
6. Determining the Empirical Formula from Combustion Analysis#
Notes:
To determine the empirical formula of a compound from Combustion
Analysis, we need to take the following steps:
- Determine the mass of the sample;
- burn the sample in oxygen;
- determine the masses of the combustion products;
- determine the number of moles of each combustion product and then use the atomic masses of elements to calculate the masses of elements other than oxygen in the original sample;
- find the mass of oxygen by finding the difference between the total mass of the organic compound and the mass of all other elements;
- use element percentage to calculate moles of C, H, N, S in a 100 g sample;
- divide the moles of each element by the moles of element present in the smallest amount;
- multiply by nonintegral ratios as necessary to give small whole numbers.
Example#
Complete combustion of a 10.5 g sample of organic compound in oxygen yielded 33g of CO2 and 13.5g of H2O. Determine the empirical formula of the organic compound.
Given: mass of sample and mass of combustion products
Asked for: empirical formula
Strategy:
A. Use the masses and molar masses of the combustion products, CO2 and H2O, to calculate the masses of carbon and hydrogen present in the original sample of organic compound.
B. Find the mass of oxygen by finding the difference between the total mass of the organic compound and the mass of carbon and hydrogen.
С. Use those masses and the molar masses of the elements to calculate the empirical formula of the organic compound.
Solution:
A. Upon combustion, 1 mol of CO2 is produced for each mole of carbon atoms in the original sample. Similarly, 1 mol of H2O is produced for every 2 mol of hydrogen atoms present in the sample. The masses of carbon and hydrogen in the original sample can be calculated from these ratios, the masses of CO2 and H2O, and their molar masses. Because the units of molar mass are grams per mole, we must first convert the masses from milligrams to grams:
Find the mass of carbon:
Find the mass of hydrogen:
B. Find the mass of oxygen: m(O) = m (organic compound) - (m(C)+m(H)) = 10.5- (9.006+1.5105) = 0 (no oxygen)
C. To obtain the relative numbers of atoms of both elements present, we need to calculate the number of moles of each and divide by the number of moles of the element present in the smallest amount:
Moles C:
Moles H:
Dividing each number by the number of moles of the element present in the smaller amount gives
Thus, the organic compound contains a 1:2 ratio of moles of carbon to moles of hydrogen: CH2. It is an empirical formula of the organic compound. In fact, the molecular formula of the organic compound is C3H6, which is consistent with our results.
Answer: The empirical formula is CH2.
7. Determining the Molecular Formula from Empirical Formula#
Calculate the molecular formula of an unknown compound if its chemical analysis shows that it contains 20% carbon, 53.3% sulfur, and 26.7% oxygen by mass, and its experimentally determined molar mass is 120.008 g/mol.
Given: percent composition and molar mass
Asked for: molecular formula
Strategy:
A. Assume 100 g of unknown compound. From the percentages given, calculate the empirical formula of the unknown compound.
B. Calculate the formula mass and then divide the experimentally determined molar mass by the formula mass. This gives the number of formula units present.
C. Multiply each subscript in the empirical formula by the number of formula units to give the molecular formula.
Solution:
A. We begin by dividing the mass of each element in 100.0 g of unknown compound (20 g of carbon, 53.3 g of sulfur, 26.7 g of oxygen) by its molar mass. This gives the number of moles of each element in 100 g of unknown compound.
These results are fairly typical of actual experimental data. None of the atomic ratios is exactly integral but all are within 5% of integral values. It is reasonable to assume that such small deviations from integral values are due to minor experimental errors, so round to the nearest integer. The empirical formula of the unknown compound is thus CSO.
B. To determine the actual molecular formula, we must divide the experimentally determined molar mass by the formula mass. The formula mass is calculated by dividing the measured molar mass of the unknown compound (60.004 g/mol) by the calculated formula mass.
C. There are two CSO formula units in unknown compound, so the molecular formula must be (CSO)2= C2S2O2.
Answer: C2S2O2
8. Chemical Equations#
Notes:
A chemical reaction changes only the distribution of atoms, not the number of atoms.
Steps in Balancing a Chemical Equation
- Identify the most complex substance.
- Beginning with that substance, choose an element that appears in only one reactant and one product, if possible. Adjust the coefficients to obtain the same number of atoms of this element on both sides.
- Balance polyatomic ions (if present) as a unit.
- Balance the remaining atoms, usually ending with the least complex substance and using fractional coefficients if necessary. If a fractional coefficient has been used, multiply both sides of the equation by the denominator to obtain whole numbers for the coefficients.
- Count the numbers of atoms of each kind on both sides of the equation to be sure that the chemical equation is balanced.
Example 1.#
The balanced chemical equation for the combustion of propanol in the laboratory is as follows: C3H8(g)+5O2(g) → 3CO2(g)+4H2O(l)
Construct a table showing how to interpret the information in this equation in terms of
- a single molecule of propanol.
- moles of reactants and products.
- grams of reactants and products represented by 1 mol of propanol.
- numbers of molecules of reactants and products represented by 1 mol of propanol.
Given: balanced chemical equation
Asked for: molecule, mole, and mass relationships
Strategy:
A. Use the coefficients from the balanced chemical equation to determine both the molecular and mole ratios.
B. Use the molar masses of the reactants and products to convert from moles to grams.
C. Use Avogadro’s number to convert from moles to the number of molecules.
Solution:
This equation is balanced as written: each side has 3 carbon atoms, 10 oxygen atoms, and 8 hydrogen atoms. We can therefore use the coefficients directly to obtain the desired information.
A. One molecule of propanol reacts with 5 molecules of O2 to yield 3 molecules of CO2 and 4 molecules of H2O.
B. One mole of propanol reacts with 5 mol of O2 to yield 3 mol of CO2 and 4 mol of H2O.
C. To interpret the equation in terms of masses of reactants and products, we need their molar masses and the mole ratios from part b. The molar masses in grams per mole are as follows: propanol, 60.004; O2, 31.9988; CO2, 44.010; and H2O, 18.015.
One mole of propanol contains Avogadro’s number (6.022 × 1023) of propanol molecules.
Thus 6.022 × 1023 propanol molecules react with (5 × 6.022 × 1023) = 11.6242 × 1023oxygen molecules to yield (3 × 6.022 × 1023) = 18.066 × 1023 molecules of CO2 and (4 × 6.022 × 1023) = 24.088 × 1023 H2O.
In tabular form:
| C3H8(g) | + | 5O2(g) | → | 3CO2(g) | + | 4H2O(l) | |
| a. | 1 molecule | 5 molecules | 3 molecules | 4 molecules | |||
| b. | 1 mol | 5 mol | 3 mol | 4 mol | |||
| c. | 60.004 g | 159.994 g | 132.03 g | 72.06 g | |||
| d. | 6.022 × 1023 molecules | 1.16242 × 1024 molecules | 1.8066 × 1024 molecules | 2.4088 × 1024 molecules |
Example 2.#
The reaction of the nonane C9H20 with oxygen gives carbon dioxide and water. Write and balance the equation for this reaction.
Given: reactants and products
Asked for: balanced chemical equation
Strategy:
A. Identify the products and the reactants and then write the unbalanced chemical equation.
B. Follow the steps for balancing a chemical equation.
Solution:
Identify the most complex substance.
We will assume initially that the final balanced chemical equation contains 1 molecule or formula unit of C9H20.
Adjust the coefficients. Try to adjust the coefficients of the molecules on the other side of the equation to obtain the same numbers of atoms on both sides. Because one molecule of n-nonane contains 9 carbon atoms, we need 9CO2 molecules, each of which contains 1 carbon atom, on the right side:
C9H20+O2→9CO2+H2O
Balance polyatomic ions as a unit. There are no polyatomic ions to be considered in this reaction.
Balance the remaining atoms. Because one molecule of n-nonane contains 20 hydrogen atoms, we need 10H2O molecules, each of which contains 2 hydrogen atoms, on the right side:
C9H20+ O2→ 9CO2+10H2O
The carbon and hydrogen atoms are now balanced, but we have 28 oxygen atoms on the right side and only 2 oxygen atoms on the left. We can balance the oxygen atoms by adjusting the coefficient in front of the least complex substance, O2, on the reactant side:
C9H20+ 14O2→ 9CO2+10H2O
Check your work. The equation is now balanced, and there are no fractional coefficients: there are 9 carbon atoms, 20 hydrogen atoms, and 28 oxygen atoms on each side.Always check to be sure that a chemical equation is balanced.
Сheck yourself:
1.What changes during a chemical reaction?
2. What factors determine whether a chemical equation is balanced?
3. What information can be obtained from a balanced chemical equation? Does a balanced chemical equation give information about the rate of a reaction?
4.How does a balanced chemical equation agree with the law of definite proportions?
5.What is the difference between P4 and 4P? Use this example to explain why subscripts in a formula must not be changed.
Explain a solution strategy using key expressions
8.1 Converting between Masses of Reactant and Product#
Notes:
The method of converting the mass of any reagent or product into the mass of any other reagent or product using a balanced chemical equation contains the general steps:
- Convert the mass of one substance (substance A) to the corresponding number of moles using its molar mass.
- From the balanced chemical equation, obtain the number of moles of another substance (B) from the number of moles of substance A using the appropriate mole ratio (the ratio of their coefficients).
- Convert the number of moles of substance B to mass using its molar mass. It is important to remember that some species are in excess by virtue of the reaction conditions. For example, if a substance reacts with the oxygen in air, then oxygen is in obvious (but unstated) excess.
Converting amounts of substances to moles—and vice versa—is the key to all stoichiometry problems, whether the amounts are given in units of mass (grams or kilograms), weight (pounds or tons), or volume (liters or gallons).
The molar masses of the reactants and the products are used as conversion factors so that you can calculate the mass of product from the mass of reactant and vice versa.
Example#
Calculate how much sodium is needed to react with 160 g of oxygen.
Given: reactants, products, and mass of one reactant
Asked for: mass of another reactant
Strategy:
A. Write the balanced chemical equation for the reaction.
B. Convert mass of oxygen to moles. From the mole ratio in the balanced chemical equation, determine the number of moles of sodium required. Then convert the moles of sodium to the equivalent mass in g.
Solution:
A. We first use the information given to write a balanced chemical equation. Because we know the identity of both the reactants and the product, we can write the reaction as follows:
B. Using the molar mass of O2 (32.00 g/mol to four significant figures) we can calculate the number of moles of O2 contained in this mass of O2
Answer: 459.6 g of sodium is needed to react with 160 g of oxygen
Сheck yourself:
Explaine the law of mass conservation of substances of M. Lomonosov
Mass balance assumes that the total mass of reactants is equal to the total mass of products. Is this a chemically valid practice? Explain your answer.
Given the equation 4Na(s)+O2(g) →2Na2O(s). Is it correct to say that 26 g of sodium will react with 26 g of oxygen to produce 52 g of sodium oxide?
What does it mean to say that a reaction is STOICHIOMETRIC?
Is it possible for the percent yield to be greater than the theoretical yield? Justify your answer.
Explain a solution strategy using key expressions.
9. Limiting Reactants#
Notes:
To determine the mass of the reaction product, if the masses of two reactants are known, we need:
- Determine the number of moles of each reactant.
- Compare the mole ratio of the reactants with the ratio in the balanced chemical equation to determine which reactant is limiting.
- Calculate the number of moles of product that can be obtained from the limiting reactant.
- Convert the number of moles of product to mass of product.
Example#
Given 20 g each of ammonia and hydrogen chloride. How many grams of ammonium chloride can be prepared from this reaction?
Given: reactants, products, and mass of reactants
Asked for: mass of product
Strategy:
A. Balance the chemical equation for the reaction.
B. Use each molar mass to convert from mass to moles.
C. Using mole ratios, determine which substance is the limiting reactant. After identifying the limiting reactant, use mole ratios based on the number of moles of limiting reactant to determine the number of moles of the product.
D. Convert from moles of product to the mass of the product.
Solution:
We always begin by writing the balanced chemical equation for the reaction:
Answer: mass of ammonium chloride is 29.346 g
Сheck yourself:
Write a balanced chemical equation for each reaction and then determine which reactant is in excess.
a) 28gH2SO3 + 16,2gZnO = ZnSO3 + H2O
b) 5.6LN2 +11.2LH2 =2NH3
Explain a solution strategy using key expressions.
10. Percent Yield#
Notes:
The amount of product calculated by the reaction equation represents the theoretical yield, which is the amount you would get if the reaction went perfectly and your product purification method was 100% effective. In fact, you almost always get less product than theoretically possible due to mechanical losses (such as spills), separation procedures that are not 100% effective, competing reactions that form unwanted products, and reactions that just don't pass all the way to completion, resulting in a mixture of products and reagents. Thus, the actual yield, the measured mass of the products resulting from the reaction, is almost always less than the theoretical yield (often much less). The percentage yield of the reaction is the ratio of the actual yield to the theoretical yield multiplied by 100 to get a percentage:
If 160 g of CuO is heated with 2g hydrogen, 48 g of a pure copper is obtained. What is the percent yield?
Given: masses of reactants and product
Asked for: percent yield
Strategy:
A. Write the balanced chemical equation.
B. Convert from mass of reactants and product to moles using molar masses and then use mole ratios to determine which is the limiting reactant. Based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield.
C. Calculate the percent yield by dividing the actual yield by the theoretical yield and multiplying by 100.
Solution:
A. From the formulas given for the reactants and the products, we see that the chemical equation is balanced as written. According to the equation, 1 mol of each reactant combines to give 1 mol of product plus 1 mol of water.
B. To determine which reactant is limiting, we need to know their numbers of mol:
C. The actual yield was only 48 g of cupper, so the percent yield was
Answer: The actual yield of copper is 76.065%.
Сheck yourself:
Determine the percent yield of each reaction. Be sure that the chemical equations are balanced. Assume that any reactants for which amounts are not given are in excess.
a) S + H2SO4 = SO2 + H2O 3.20 g of sulfur gives 5.4 g of sulfur dioxide
b) S + NaOH = Na2S + Na2SO3 H2O 4.0 g of sodium hydroxide produces 4.2 g of sodium sulfide
Explain a solution strategy using key expressions.
11. Atom economy#
Notes:
No atoms are gained or lost in a chemical reaction. However, some atoms in the reactants may not end up in the desired product. They instead form other products, and so are regarded as by-products.
The atom economy of a reaction is a measure of the amount of starting materials that end up as useful products. It is important for sustainable development and for economic reasons to use reactions with high atom economy. The percentage atom economy of a reaction is calculated using this equation:
Example#
Hydrogen can be manufactured by reacting methane with steam:
CH4(g) + H2O(g) → 3H2(g) + CO(g). Using a balanced equation, determine the atom economy for the reaction.
Given: balanced equation
Asked for: atom economy for the reaction relative to hydrogen
Strategy:
- We calculate the molecular mass of all compounds
- Calculate the atom economy for the reaction
Solution:
Mr(CH4) = 16;
Mr(H2O) = 18;
Mr(H2) =2;
total Mr of desired product = 3∙2 = 6;
Mr(СO) =28
Answer: 17.6%
Сheck yourself:
What is the atom economy of the reaction below in producing water?
a) CH4(g) + 2O2(g) => 2H2O(g) + CO2(g)
b) 2H2+O2 = 2H2O
Explain a solution strategy using key expressions.
12. Ionic Equations#
Notes:
The chemical equation for a reaction in solution can be written in three ways. The overall chemical equation shows all the substances present in their undissociated forms; the complete ionic equation shows all the substances present in the form in which they actually exist in solution; and the net ionic equation is derived from the complete ionic equation by omitting all spectator ions, ions that occur on both sides of the equation with the same coefficients. Net ionic equations demonstrate that many different combinations of reactants can give the same net chemical reaction.
Example 1.#
Write the overall chemical equation, the complete ionic equation, and the net ionic equation for the reaction of aqueous aluminum chloride with aqueous potassium phosphate to give solid aluminum phosphate and a solution of potassium chloride.
Given: reactants and products
Asked for: overall, complete ionic, and net ionic equations
Strategy:
- Write and balance the overall chemical equation.
- Write all the soluble reactants and products in their dissociated form to give the complete ionic equation.
- Then cancel species that appear on both sides of the complete ionic equation to give the net ionic equation.
Solution:
1.From the information given, we can write and balance the overall chemical equation:
AlCl3(aq)+K3PO4(aq)→AlPO4(s)+3KCl(aq)
This is the overall balanced chemical equation for the reaction, showing the reactants and products in their undissociated form. To obtain the complete ionic equation, we write each soluble reactant and product in dissociated form:
Al3+(aq) +3Cl−(aq) +3K+(aq)+PO43−(aq)→AlPO4(s)+3K+(aq)+3Cl−(aq)
The three Cl−(aq)ions and the three 3K+(aq)ions that appear on both sides of the equation are spectator ions that can be canceled to give the net ionic equation:
Al3+(aq)+2PO43−(aq)→ AlPO4(s)
Example 2.#
Write the overall chemical equation, the complete ionic equation, and the net ionic equation for the reaction of aqueous sodium carbonate with aqueous hydrochloric acid to give solid silver phosphate and a solution of sodium fluoride.
Given: reactants and products
Asked for: overall, complete ionic, and net ionic equations
Strategy:
- Write and balance the overall chemical equation.
- Write all the soluble reactants and products in their dissociated form to give the complete ionic equation.
- Then cancel species that appear on both sides of the complete ionic equation to give the net ionic equation.
Solution:
- overall chemical equation:
Na2CO3(aq)+2HCl(aq)→2NaCl(aq)+CO2(g) + H2O(l) - complete ionic equation:
2Na+(aq) + CO32-(aq) + 2H+(aq) + 2Cl-(aq) →2Na+(aq) + 2Cl-(aq) + CO2(g) + H2O(l) - net ionic equation:
2H+(aq) + CO32-(aq) →CO2(g) + H2O(l)
Сheck yourself:
Write the overall chemical equation, the complete ionic equation, and the net ionic equation for the reactions:
a) K2SO3 + HNO3
b) Ba(OH)2 + HNO3
Explain a solution strategy using key expressions.
13. Solution Concentrations#
Notes:
The most common unit of concentration is molarity, which is also the most useful for calculations involving the stoichiometry of reactions in solution. The molarity (M) of a solution is the number of moles of solute present in exactly 1L of solution. Molarity is also the number of millimoles of solute present in exactly 1 mL of solution:
The units of molarity are moles per liter of solution (mol/L), abbreviated as M. In chemical notation, square brackets around the name or formula of the solute represent the concentration of a solute. So
is read as “the concentration of sodium hydroxide is 1.00 molar.” Solutions of known concentration can be prepared either by dissolving a known mass of solute in a solvent and diluting to a desired final volume or by diluting the appropriate volume of a more concentrated solution (a stock solution) to the desired final volume.
Example 1.#
Calculate the number of moles of sulfuric acid (H2SO4) in 3.00 L of 0.150 M H2SO4.
Given: identity of solute and volume and molarity of solution
Asked for: amount of solute in moles
Strategy:
Use the formula for finding the number of moles
n = С∙V
Solution:
n(H2SO4) = 0.150∙3.00 = 0.45M
Answer: n(H2SO4) = 0.45M
Example 2.#
The solution contains 25.0 g of copper (II) sulfate pentahydrate, CuSO4·5H2O, in enough ethanol to make exactly 800 mL of solution. What is the molar concentration of CuSO4·5H2O?
Given: mass of solute and volume of solution
Asked for: concentration (M)
Strategy: To find the number of moles of CuSO4·5H2O, divide the mass of the compound by its molar mass. Calculate the molarity of the solution by dividing the number of moles of solute by the volume of the solution in liters.
Solution:
The molar mass of CuSO4·5H2O is 250 g/mol.
Therefore, moles CuSO4·5H2O = () = 0.1mol
The volume of the solution in liters is
volume=800mL=0.800L volume
Molarity is the number of moles of solute per liter of solution, so the molarity of the solution is
Answer: [CuSO4·5H2O] = 0.125M
Example 3.#
Calculate the mass of acetic acid needed to prepare 500 ml of its 0.2 M solution. Acetic acid has a molar mass of 60 g/mol.
Given: molarity, volume, and molar mass of solute
Asked for: mass of acetic acid
Strategy:
A. Calculate the number of moles of acetic acid contained in the indicated volume of dilute solution by multiplying the volume of the solution by its molarity.
B. To determine the mass of acetic acid multiply its number of moles by the molar mass
Solution:
A. We begin by using equation n = C∙V to calculate the number of moles of acetic acid contained in 500 mL of the solution:
n (acetic acid) = 0.2M∙0.5L=0.1 mol
B. Now we must determine the mass of acetic acid:
m(acetic acid )=n∙Molar mass
m(acetic acid )=0.1mol∙60g/mol=6 g
Answer: m(acetic acid )=6 g
Example 4.#
What volume of a 2.0 M KOH stock solution is necessary to prepare 400 mL 0.1 M KOH?
Given: volume and molarity of dilute solution
Asked for: volume of stock solution
Strategy:
A. Calculate the number of moles of potassium hydroxide contained in the indicated volume of dilute solution by multiplying the volume of the solution by its molarity.
B. To determine the volume of stock solution needed, divide the number of moles of potassium hydroxide by the molarity of the stock solution.
Solution: Determine the number of moles of potassium hydroxide in 400 ml of 0.1 M solution:
B. We must now determine the volume of the 2.00 M stock solution that contains this amount of potassium hydroxide:
Answer: volume of a 2.0 M KOH stock solution is 0.2 L
Example 5.#
What are the concentrations of all species derived from the solutes in these aqueous solutions?
- 1.2 M Na2SO4
- 1.5 M Al(NO3)3
- 2.5 M CH3COCH3
Given: molarity
Asked for: concentrations
Strategy:
A. Classify each compound as either a strong electrolyte or a nonelectrolyte.
B. If the compound is a nonelectrolyte, its concentration is the same as the molarity of the solution. If the compound is a strong electrolyte, determine the number of each ion contained in one formula unit. Find the concentration of each species by multiplying the number of each ion by the molarity of the solution.
Solution:
- Sodium sulfate is an ionic compound that is a strong electrolyte in aqueous solution:
Na2SO4(aq) ↔2Na+(aq) + SO42-(aq)
One formula unit of Na2SO4 produces two Na+ ions and one SO42-(aq) ion, so a 1.2 M Na2SO4 solution contains 2.4 M Na+ and 1.2 M SO42- that is, [Na+] 2.4 M and [SO42- ] = 1.2 M. - Aluminum nitrate is an ionic compound that is a strong electrolyte in aqueous solution:
Al(NO3)3(aq) ↔Al3+(aq) + 3NO3-(aq)
One formula unit of Al(NO3)3 produces one Al3+ ion and three NO3- ions, so a 1.5 M Al(NO3)3solution contains 1.5 M Al3+ and 3 M NO3- that is, [Al3+] 1.5 M and [NO3-] = 4.5 M. - The formula CH3COCH3 represents ketone acetone. Ketone are covalent compounds that dissolve in water to give solutions of neutral molecules. Thus ketone are nonelectrolytes. The only solute species in solution is therefore CH3COCH3 molecules, so [CH3COCH3] = 2.5 M.
Answer: [CH3COCH3] = 2.5 M
Сheck yourself:
- Calculate the number of grams of solute in 2.00 L of each solution.
a) 2.56 M K2CO3
b) 0.85M Ca(NO3)2- What is the concentration of each species present in the following aqueous solutions?
a) 0.25 mol of sodium nitrate in 250 mL of solution
b) 1.8 mol of nitric acid in 650 mL of solutionExplain a solution strategy using key expressions
14. Units for Concentration#
Notes:
Different units are used to express the concentrations of a solution depending on the application. The concentration of a solution is the quantity of solute in a given quantity of solution. It can be expressed in several ways: molarity (moles of solute per liter of solution); mole fraction, the ratio of the number of moles of solute to the total number of moles of substances present; mass percentage, the ratio of the mass of the solute to the mass of the solution times 100; parts per thousand (ppt), grams of solute per kilogram of solution; parts per million (ppm), milligrams of solute per kilogram of solution; parts per billion (ppb), micrograms of solute per kilogram of solution; and molality (m), the number of moles of solute per kilogram of solvent.
| Unit | Definition | Application |
| molarity (M) | moles of solute/liter of solution (mol/L) | Used for quantitative reactions in solution and titrations; mass and molecular mass of solute and volume of solution are known. |
| mole fraction (X) | moles of solute/total moles present (mol/mol) | Used for partial pressures of gases and vapor pressures of some solutions; mass and molecular mass of each component are known. |
| molality (m) | moles of solute/kg of solvent (mol/kg) | Used in determining how colligative properties vary with solute concentration; masses and molecular mass of solute are known. |
| mass percentage (%) | [mass of solute (g)/mass of solution (g)] × 100 | Useful when masses are known but molecular masses are unknown. |
| parts per thousand (ppt) | [mass of solute/mass of solution] × 103 (g solute/kg solution) | Used in the health sciences, ratio solutions are typically expressed as a proportion, such as 1:1000. |
| parts per million (ppm) | [mass of solute/mass of solution] × 106 (mg solute/kg solution) | Used for trace quantities; masses are known but molecular masses may be unknown. |
| parts per billion (ppb) | [mass of solute/mass of solution] × 109 (μg solute/kg solution) | Used for trace quantities; masses are known but molecular masses may be unknown. |
| * The molarity of a solution is temperature dependent, but the other units shown in this table are independent of temperature. | ||
Example#
For 120 ml of 20% sulfuric acid solution (density 0.72g / ml) calculate
- the mass of the solution
- the mole fraction
- the molarity
- the molality
Given: volume, percent and density
Asked for: mole fraction, molarity, and molality
Strategy:
A. Use the density of the solution to calculate the mass of 120 mL solution.
B. Convert grams of solute and solvent to moles of solute and solvent. Calculate the mole fraction of solute by dividing the moles of solute by the total number of moles of substances present in solution.
С. Calculate the molarity of the solution: moles of solute per liter of solution. Determine the molality of the solution by dividing the number of moles of solute by the kilograms of solvent.
Solution:
А. m= ρ∙V
В. To calculate the mole fraction of sulfuric acid in the solution, we need to know the mass of sulfuric acid and the number moles of both sulfuric acid and water. The mass of sulfuric acid is
С. The molarity of the solution is the number of moles of sulfuric acid per liter of solution. We already know the number of moles of sulfuric acid, so the molarity is
Answer:
- mass of the solution = 86.4g
- mole fraction = 0.0438
- molarity = 1.47M
- molality = 2.546 mol/kg
Сheck yourself:
If 2 liters of a 5% sodium chloride solution (density 0.18g / L) is given, determine its molarity and molality.
Explain a solution strategy using key expressions.
15. pH and pOH#
Notes:
We can convert between [H+] and pH using the following equations:
pH = -log[H+]
[H +] = 10-pH
We can convert between [OH-] and pOH using the following equations:
pOH = -log [OH-]
[OH-] = 10-pOH
For any aqueous solution at 25∘C:
pH + pOH = 14
For every factor of 10 INCREASE in concentration of [H+], pH, will DECREASE by 1 unit, and vice versa. Both acid strength and concentration determine [H+] and pH.
Example 1. Calculating the pH of a strong base solution#
If we use 2 mol of KOH to make of 4.0L an aqueous solution at 25∘C, what is the pH of this solution?
Given: amount of substance, volume solution
Asked for: pH of solution
Strategy:
A. Calculate the molar concentration of KOH
B. Calculate [OH-] based on the dissociation of KOH
C. Calculate pOH from [OH-]
D. Calculate pH from pOH using pH + pOH = 14
Solution:
A. Molar concentration is equal to moles of solute per liter of solution:
The concentration of KOH in the solution is 0.5 M
B. Because KOH is a strong base, it dissociates completely into its constituent ions in aqueous solution: KOH(ag)↔K+(ag) + OH-(ag)
This balanced equation tells us that every mole of KOH produces one mole of OH- in aqueous solution. Therefore, we have the following relationship between [KOH] and [OH-]:
[KOH] =[OH-]=0.5M
C. Now that we know the concentration of [OH-], we can calculate pOH using pOH = -log [OH-] = - log (0.5) = 0.3
D. We can calculate pH from pOH using pH + pOH = 14
We can substitute the value of pOH we found in «С» to find the pH:
pH = 14 – pOH
pH = 14 –0.3 = 13.7
Example 2. Calculating the pH of a weak acid solution#
Calculate the pH of 0.200 moldm-3 ethanoic acid, CH3COOH. (Ka = 1.74 × 10-5mol dm-3)
Step 1 Write the equilibrium expression for the reaction.
Step 2 Enter the values into the expression.
Step 3 Rearrange the equation.
Step 4 Take the square root.
Step 5 Calculate pH.
Answer: pH=1.73
Сheck yourself:
- If we use 3 mol of NaOH to make of 0.6L an aqueous solution at 40∘C, what is the pH of this solution?
- Calculate the pH of 0.100 moldm-3 ethanoic acid, CH3COOH. (Ka = 1.74 × 10-5mol dm-3)
Explain a solution strategy using key expressions.
16. Calculating the pH of a Buffer#
Notes:
Buffers are solutions that resist a change in pH after adding an acid or a base. Buffers contain a weak acid (HA) and its conjugate weak base (A−). Adding a strong electrolyte that contains one ion in common with a reaction system that is at equilibrium shifts the equilibrium in such a way as to reduce the concentration of the common ion. The shift in equilibrium is called the common ion effect. Buffers are characterized by their pH range and buffer capacity. The useful pH range of a buffer depends strongly on the chemical properties of the conjugate weak acid–base pair used to prepare the buffer (the Ka or Kb), whereas its buffer capacity depends solely on the concentrations of the species in the solution. The pH of a buffer can be calculated from the concentrations of the weak acid and the weak base used to prepare it, the concentration of the conjugate base and conjugate acid, and the pKa or pKb of the weak acid or weak base.
An alternative method frequently used to calculate the pH of a buffer solution is based on a rearrangement of the equilibrium equation for the dissociation of a weak acid. The simplified ionization reaction is HA⇌H+ +A− for which the equilibrium constant expression is as follows:
If [base] = [acid] for a buffer, then pH = pKa. Changing this ratio by a factor of 10 either way changes the pH by ±1 unit.
Because no single buffer system can effectively maintain a constant pH value over the physiological range of approximately 5 to 7.4, biochemical systems use a set of buffers with overlapping ranges. The most important of these is the CO2/HCO3− system, which dominates the buffering action of blood plasma.
Example 1.#
What is the pH of a solution that contains 0.03M NH3 and 0.04M. (pKb = 4.76)
Given: concentration of base, conjugate acid, and pKb
Asked for: pH
Strategy:
Substitute values into the Henderson-Hasselbalch equation to calculate the pH.
Solution:
Inserting the given values into the equation . Thus pKa for the ammonium ion is
. Substituting this pKa value into the Henderson-Hasselbalch equation,
Answer: pH=9.11
Example 2.#
The buffer solution contained 0.135 M HCO2H and 0.215 M HCO2Na and had a pH of 3.95.
- What is the final pH if 5.00 mL of 1.00 M HCl are added to 100 mL of this solution?
- What is the final pH if 5.00 mL of 1.00 M NaOH are added?
Given: composition and pH of buffer; concentration and volume of added acid or base
Asked for: final pH
Strategy:
1.
A. Calculate the amounts of formic acid and formate present in the buffer solution. Then calculate the amount of acid or base added.
B. Construct a table showing the amounts of all species after the neutralization reaction. Use the final volume of the solution to calculate the concentrations of all species. Finally, substitute the appropriate values into the Henderson-Hasselbalch equation to obtain the pH.
Solution:
The added HCl (a strong acid) or NaOH (a strong base) will react completely with formate (a weak base) or formic acid (a weak acid), respectively, to give formic acid or formate and water. We must therefore calculate the amounts of formic acid and formate present after the neutralization reaction.
A. We begin by calculating the millimoles of formic acid and formate present in 100 mL of the initial pH 3.95 buffer:
The millimoles of H+ in 5.00 mL of 1.00 M HCl is as follows:
B. Next, we construct a table of initial amounts, changes in amounts, and final amounts:
| [HCO2−] | [H+] | [HCO2H] | |
| initial | 21.5 mmol | 5.00 mmol | 13.5 mmol |
| change | −5.00 mmol | −5.00 mmol | +5.00 mmol |
| final | 16.5 mmol | ∼0 mmol | 18.5 mmol |
Because the total volume appears in both the numerator and denominator, it cancels. We therefore need to use only the ratio of the number of millimoles of the conjugate base to the number of millimoles of the weak acid. So
2.
The number of millimoles of OH− in 5.00 mL of 1.00 M NaOH is as follows:
With this information, we can construct a table of initial amounts, changes in amounts, and final amounts.
| [HCO2H] | [OH-] | [HCO2−] | |
| initial | 13.5 mmol | 5.00 mmol | 21.5 mmol |
| change | −5.00 mmol | −5.00 mmol | +5.00 mmol |
| final | 8.5 mmol | ∼0 mmol | 26.5 mmol |
Answer: pH=3.7 pH=4.24
Сheck yourself:
What is the pH of a solution that contains 0.025M NH3 and 0.15M NH4Cl (pKb = 4.76)
What is the final pH if 15.00 mL of 0.020 M HCl are added to 0.85 mL of the buffered solution?
What is the final pH if 8.00 mL of 0.120 M NaOH are added to 92.0 mL of the buffered solution?
Explain a solution strategy using key expressions
17. Applying the Ideal Gas Law#
Notes:
The empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into the ideal gas law, PV = nRT. The proportionality constant, R, is called the gas constant and has the value 0.08206 , 8.3145
or 1.9872
, depending on the units used. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. Standard temperature and pressure (STP) is 0°C and 1 atm. The volume of 1 mol of an ideal gas at STP is 22.41 L, the standard molar volume. All of the empirical gas relationships are special cases of the ideal gas law in which two of the four parameters are held constant. The ideal gas law allows us to calculate the value of the fourth quantity (P, V, T, or n) needed to describe a gaseous sample when the others are known and also predict the value of these quantities following a change in conditions if the original conditions (values of P, V, T, and n) are known. The ideal gas law can also be used to calculate the density of a gas if its molar mass is known or, conversely, the molar mass of an unknown gas sample if its density is measured.
Example 1.#
How many moles of oxygen gas were needed to fill the balloon if its volume was 400L, the temperature at 25°C and the atmospheric pressure was 750 mmHg?
Given: volume, temperature, and pressure
Asked for: amount of gas
Strategy:
A. Solve the ideal gas law for the unknown quantity, in this case n.
B. Make sure that all quantities are given in units that are compatible with the units of the gas constant. If necessary, convert them to the appropriate units, insert them into the equation you have derived, and then calculate the number of moles of hydrogen gas needed.
Solution:
A. We are given values for P, T, and V and asked to calculate n. If we solve the ideal gas law for n, we obtain: n=PVRT
B. P and T are given in units that are not compatible with the units of the gas constant [0.08206 ]. We must therefore convert the temperature to kelvins and the pressure to atmospheres:
Substituting these values into the expression we derived for n, we obtain
n=PVRT=0.9868atm ∙ 400L ∙ 0.08206 298K = 9652.85 mol = 9.653 ∙ 103 mol
Answer: 9.653∙103 mol
Example 2.#
A sample of the gas containing nitrogen and oxygen at a pressure of 727 mmHg and a temperature of 18°C weighs 0.289 g in a flask with a volume of 157.0 mL. Calculate the molar mass of the gas and suggest a reasonable chemical formula for the compound.
Given: pressure, temperature, mass, and volume
Asked for: molar mass and chemical formula
Strategy:
A. Solve Equation =PRT for the molar mass of the gas and then calculate the density of the gas from the information given.
B. Convert all known quantities to the appropriate units for the gas constant being used. Substitute the known values into your equation and solve for the molar mass.
C. Propose a reasonable empirical formula using the atomic masses of nitrogen and oxygen and the calculated molar mass of the gas.
Solution:
A. Density is the mass of the gas divided by its volume:
B. We must convert the other quantities to the appropriate units before inserting them into the equation:
C. The atomic masses of N and O are approximately 14 and 16, respectively, so we can construct a list showing the masses of possible combinations:
The most likely choice is NO2 which agrees with the data. The red-brown color of smog also results from the presence of NO2 gas.
Answer: NO2
Сheck yourself:
- Calculate the number of moles in each sample at STP.
a) 224 cm3 of carbon monoxide
b) 33.6 L of sulfur dioxide- Calculate the mass of each sample at STP.
a) 1.12 L of nitrogen dioxide
b) 5.6 ml of methane- Calculate the volume in liters of each sample at STP.
a) 1.2 L at 24°C and 26.4 kPa of oxygen
b) 230 mL at −9°C and 745 mmHg of hydrogen- Calculate the density of Cl2 under each set of conditions.
a) 2.4 mol at 96 kPa and -13°C
b) 14.2 g at 0.78 atm and 18°CExplain a solution strategy using key expressions.
18. Stoichiometry Involving Gases#
Notes:
The relationship between the amounts of products and reactants in a chemical reaction can be expressed in units of moles or masses of pure substances, of volumes of solutions, or of volumes of gaseous substances. The ideal gas law can be used to calculate the volume of gaseous products or reactants as needed.
The ideal gas equation and the stoichiometry of a reaction can be used to calculate the volume of gas produced or consumed in a reaction.
Example#
What volume of NO2 (in liters) at 18°C and 755 mmHg pressure is required to produce 3.00 tons of HNO3 according to the equation: 4NO2+O2+2H2O = 4HNO3?
Given: reaction, temperature, pressure, and mass of one product
Asked for: volume of gaseous reactant
Strategy:
A. Calculate the number of moles of HNO3 in 3.00 tons. From the stoichiometric coefficients in the balanced chemical equation, calculate the number of moles of NO2 required.
B. Use the ideal gas law to determine the volume of NO2 required under the given conditions. Be sure that all quantities are expressed in the appropriate units.
Solution:
We can see from the stoichiometry of the reaction that 4 mol of NO2 is required to produce 4 mol of HNO3.
A. We begin by calculating the number of moles of HNO3 in 3.00Tn:
We next calculate the number of moles of NO2 required:
We must convert the temperature to kelvins and the pressure to atmospheres:
B. After converting all quantities to the appropriate units, we can use the ideal gas law to calculate the volume of NO2:
Answer: The answer means that 114.47∙104L of nitrogen dioxide gas are needed to produce
3 Tn of Nitric acid.
Сheck yourself:
- Complete the decomposition of a sample of sodium nitrate produced 8.5 g of sodium nitrite and oxygen gas.
What is the mass of sodium nitrate in the original sample?
What mass of oxygen is produced?
What is the volume of oxygen produced at STP?- What volume of gas would be produced at 14°C and 728 mmHg by decomposition 6.8 g of hydrogen peroxide?
Explain a solution strategy using key expressions.
19. Calorimetry#
Notes:
Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters, which measure the change in temperature when a chemical reaction is carried out.
The magnitude of the temperature change depends on the amount of heat released or absorbed and on the heat capacity of the system.
The heat capacity (C) of an object is the amount of energy needed to raise its temperature by 1°C; its units are joules per degree Celsius.
The specific heat (Cs) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°C, and the molar heat capacity (Cp) is the amount of energy needed to raise the temperature of 1 mol of a substance by 1°C.
Liquid water has one of the highest specific heats known. Heat flow measurements can be made with either a constant-pressure calorimeter, which gives ΔH values directly, or a bomb calorimeter, which operates at constant volume and is particularly useful for measuring enthalpies of combustion.
Example#
When 6.2 g of solid sodium hydroxide is dissolved in 200.0 mL of distilled water in a calorimeter, the temperature of the liquid increases from 21.0°C to 28.4°C. The density of water in this temperature range averages 0.9978. What is ΔHsoln (in kilojoules per mole)? Assume that the calorimeter absorbs a negligible amount of heat and, because of the large volume of water, the specific heat of the solution is the same as the specific heat of pure water.
Given: mass of substance, volume of solvent, and initial and final temperatures
Asked for: ΔHsoln
Strategy:
A. Calculate the mass of the solution from its volume and density and calculate the temperature change of the solution.
B. Find the heat flow that accompanies the dissolution reaction by substituting the appropriate values into
C. Use the molar mass of NaOH to calculate ΔHsoln.
Solution:
A. To calculate ΔHsoln, we must first determine the amount of heat released in the calorimetry experiment. The mass of the solution is
the temperature change is (28.4°C − 21.0°C) = +7.4°C.
B. Because the solution is not very concentrated (approximately 0.775 M), we assume that the specific heat of the solution is the same as that of water. The heat flow that accompanies dissolution is thus
The temperature of the solution increased because heat was absorbed by the solution (q > 0). Where did this heat come from? It was released by NaOH dissolving in water. From
Equation we see that ΔHrxn = −qcalorimeter = −7.371 k
This experiment tells us that dissolving 6.2 g of NaOH in water is accompanied by the release of 7.371 kJ of energy. Because the temperature of the solution increased, the dissolution of NaOH in water must be exothermic.
C. The last step is to use the molar mass of NaOH to calculate ΔHsoln—the heat released when dissolving 1 mol of NaOH:
Answer: 47.55 kJ
Сheck yourself:
When 0.56 g of solid calcium oxide are dissolved in 100.0 mL of distilled water in a calorimeter, the temperature of the liquid increases from 17.0°C to 22.4°C. The density of water in this temperature range averages 0.9978. What is ΔHsoln (in kilojoules per mole)?
Explain a solution strategy using key expressions.
20. Hess's Law#
Notes:
Hess's law: The overall enthalpy change for a series of reactions is the sum of the enthalpy changes for the individual reactions.
When using Hess’s law to calculate the value of ΔH for a reaction, follow this procedure:
1) Identify the equation whose ΔH value is unknown and write individual reactions with known ΔH values that, when added together, will give the desired equation.
2) Arrange the chemical equations so that the reaction of interest is the sum of the individual reactions.
3) If a reaction must be reversed, change the sign of ΔH for that reaction. Additionally, if a reaction must be multiplied by a factor to obtain the correct number of moles of a substance, multiply its ΔH value by that same factor.
4) Add together the individual reactions and their corresponding ΔH values to obtain the reaction of interest and the unknown ΔH.
Example#
When carbon is burned with limited amounts of oxygen gas (O2), carbon monoxide (CO) is the main product:
(1) 2C(s)+O2(g)→2CO(g) ΔH=−221.0kJ
When carbon is burned in excess O2, carbon dioxide (CO2) is produced:
(2) C(s)+O2(g)→CO2(g) ΔH=−393.5kJ
Use this information to calculate the enthalpy change per mole of CO for the reaction of CO with O2 to give CO2.
Given: two balanced chemical equations and their ΔH values
Asked for: enthalpy change for a third reaction
Strategy:
A. After balancing the chemical equation for the overall reaction, write two equations whose ΔH values are known and that, when added together, give the equation for the overall reaction. (Reverse the direction of one or more of the equations as necessary, making sure to also reverse the sign of ΔH.)
B. Multiply the equations by appropriate factors to ensure that they give the desired overall chemical equation when added together. To obtain the enthalpy change per mole of CO, write the resulting equations as a sum, along with the enthalpy change for each.
Solution:
A. We begin by writing the balanced chemical equation for the reaction of interest:
(3) CO(g)+0.5O2(g)→CO2(g) ΔHrxn=?
There are at least two ways to solve this problem using Hess’s law and the data provided. The simplest is to write two equations that can be added together to give the desired equation and for which the enthalpy changes are known. Observing that CO, a reactant in Equation 3, is a product in Equation 1, we can reverse Equation (1) to give
2CO(g) →2C(s)+O2(g) ΔH=+221.0kJ
Because we have reversed the direction of the reaction, the sign of ΔH is changed. We can use Equation 2 as written because its product, CO2, is the product we want in Equation 3:
C(s) + O2(g) →CO2(s) ΔH=−393.5kJ
B. Adding these two equations together does not give the desired reaction, however, because the numbers of C(s) on the left and right sides do not cancel. According to our strategy, we can multiply the second equation by 2 to obtain 2 mol of C(s) as the reactant:
C(s) +2O2(g)→2CO2(s) ΔH=−787.0kJ
Writing the resulting equations as a sum, along with the enthalpy change for each, gives
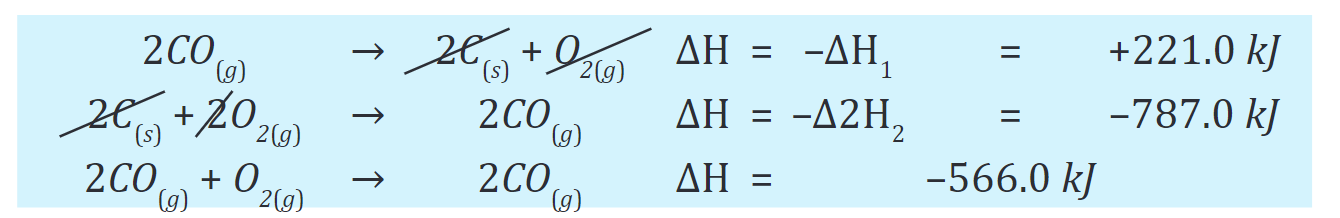 Note that the overall chemical equation and the enthalpy change for the reaction are both for the reaction of 2 mol of CO with O2, and the problem asks for the amount per mole of CO. Consequently, we must divide both sides of the final equation and the magnitude of ΔH by 2:
Note that the overall chemical equation and the enthalpy change for the reaction are both for the reaction of 2 mol of CO with O2, and the problem asks for the amount per mole of CO. Consequently, we must divide both sides of the final equation and the magnitude of ΔH by 2:Answer: -283.0kJ
Сheck yourself:
Self-heating cans may be used to warm drinks such as coffee. When the button on the can is pushed, a seal is broken, allowing water and calcium oxide to mix and react. The reaction produces solid calcium hydroxide and releases heat. If more water is used, the calcium hydroxide is produced as a solution instead of as a solid. The equation for the reaction is:
CaO(s) + H2O(ℓ) → Ca(OH)2(aq)
Using the following data to calculate the enthalpy change, in kJmol−1, for this reaction.
Ca(s) + ½O2(g) → CaO(s) ΔH = −635 kJmol−1
H2(g) + ½O2(g) → H2O(ℓ) ΔH = −286 kJmol−1
Ca(s) + O2(g) + H2(g) → Ca(OH)2(s) ΔH = −986 kJmol−1
Ca(OH)2(s) → Ca(OH)2(aq) ΔH = −82 kJmol−1
Explain a solution strategy using key expressions.
21. Standard Enthalpies of Reaction#
Notes:
The enthalpy of formation (ΔHf) is the enthalpy change that accompanies the formation of a compound from its elements. Standard enthalpies of formation (ΔH°f) are determined under standard conditions: a pressure of 1 atm for gases and a concentration of 1 M for species in solution, with all pure substances present in their standard states (their most stable forms at 1 atm pressure and the temperature of the measurement). The standard heat of formation of any element in its most stable form is defined to be zero. The standard enthalpy of reaction (ΔH°rxn) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its stoichiometric coefficient) minus the sum of the standard enthalpies of formation of the reactants (each multiplied by its stoichiometric coefficient)—the “products minus reactants” rule. The enthalpy of solution (ΔHsoln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure.
Example 1.#
For the formation of each compound, write a balanced chemical equation corresponding to the standard enthalpy of formation of each compound.
1) H2O(g)
2) Na2CO3(s)
Given: compound
Asked for: balanced chemical equation for its formation from elements in standard states
Strategy:
Identify the standard state for each element. Write a chemical equation that describes the formation of the compound from the elements in their standard states and then balance it so that 1 mol of product is made.
Solution:
1) Hydrogen oxide contains one atom of hydrogen and one atom of oxygen. Because the standard states of elemental hydrogen and elemental oxygen are H2(g) and O2(g), respectively, the unbalanced chemical equation is H2(g) + O2(g) → H2O(g)
Fractional coefficients are required in this case because ΔH°f values are reported for 1 mol of the product, H2O. Multiplying O2(g) by 1/2 balances the equation:
Example 2.#
Use standard enthalpies of formation table to determine the change in enthalpy for each of these reactions.
a) NaOH(s) + HCl(g) →NaCl(s) + H2O(g)
b) 2CO(g) + O2(g) →2CO2(g)
c) CH4(g) + 2O2(g) →CO2(g) + 2H2O(l)
Given: compound and ΔH°f values
Table 2. Standard enthalpies of formation
| Compound | ΔHf (kJ/mol) | Compound | ΔHf (kJ/mol) |
| CH4(g) | -74.8 | HCl(g) | -92.3 |
| CO2(g) | -393.5 | H2O2(g) | -241.8 |
| NaOH(s) | -426.7 | H2O(l) | 285.8 |
| O2 | 0 | NaCl(s) | -411.0 |
| KBr(s) | −393.8 | H2SO4(l) | −909.3 |
| CaCO3(s) | −1207.6 | CaO(s) | −634.9 |
| K2SO4(s) | −1437.8 | HBr(g) | −36.3 |
Asked for: change in enthalpy
Strategy:
After writing the balanced chemical equation for the reaction, use
ΔH°rxn = ΣΔH°f(products) −ΔH°f(reactants) and the values from the Table 2 to calculate ΔH°rxn.
Solution:
To determine the ΔH°rxn, we need the balanced chemical equation and
a) NaOH(s) + HCl(g) →NaCl(s) + H2O(g)
[(-411.0) + (-241.8)] – [(-426.7) + (-92.3)] = -133.8 kJ
b) 2CO(g) + O2(g) →2CO2(g)
[2(-393.5)] – [2(-110.5)] = -566 kJ
c) CH4(g) + 2O2(g) →CO2(g) + 2H2O(l)
[(-393.5) + 2(-285.8)] – [(-74)] = -891.1 kJ
Сheck yourself:
Calculate ΔH°rxn for each reaction. If necessary, balance the chemical equations.
2KBr(s) + H2SO4(l) → K2SO4(s) + 2HBr(g)
CaCO3(s, calcite) → CaO(s) + CO2(g)
Explain a solution strategy using key expressions.
22. Equilibrium Constant Expression#
Notes:
A large value of the equilibrium constant K means that products predominate at equilibrium; a small value means that reactants predominate at equilibrium.
Example 1.#
Write the equilibrium constant expression for the reaction.
2CO2(g) ⇌ 2CO(g)+ O2(g)
Given: balanced chemical equation
Asked for: equilibrium constant expression
Strategy:
Place the arithmetic product of the concentrations of the products (raised to their stoichiometric coefficients) in the numerator and the product of the concentrations of the reactants (raised to their stoichiometric coefficients) in the denominator.
Solution:
Example 2.#
Predict which systems at equilibrium will (a) contain essentially only products, (b) contain essentially only reactants, and (c) contain appreciable amounts of both products and reactants.
H2(g)+ I2(g) ⇌ 2HI(g) K (700 K) = 54
2CO2(g) ⇌ 2CO(g) + O2(g) K (1200 K) = 3.1×10−18
PCl5(g) ⇌ PCl3(g) + Cl2(g) K (613 K) = 97
2O3(g)⇌3O2 (g) K (298 K) =5.9×1055
Given: systems and values of K
Asked for: composition of systems at equilibrium
Strategy:
Use the value of the equilibrium constant to determine whether the equilibrium mixture will contain essentially only products, essentially only reactants, or significant amounts of both.
Solution:
Only system 4 has K >> 103, so at equilibrium it will consist of essentially only products.
System 2 has K <<center 10−3, so the reactants have little tendency to form products under the conditions specified; thus, at equilibrium the system will contain essentially only reactants.Both systems 1 and 3 have equilibrium constants in the range 103 ≥ K ≥ 10−3, indicating that the equilibrium mixtures will contain appreciable amounts of both products and reactants.
Сheck yourself:
1.Write the equilibrium constant expression for reaction
N2(g) + O2(g)⇌2NO(g)
C(s) + 2Cl2(g) = CCl4(g)
Explain a solution strategy using key expressions.
23. Reaction Order Determination#
Notes:
The Order of Reaction refers to the power dependence of the rate on the concentration of each reactant. For a first-order reaction, the rate is dependent on the concentration of a single species. Or in this case: A + B = C rate = [A][B], the order of reaction with respect to both A and B is 1. The overall order of reaction is 2 - found by adding up the individual orders. In this case: rate = [B] the reaction is first order with respect to B and zero order with respect to A, because the concentration of A doesn't affect the rate of the reaction. The reaction is first order overall (because 1 + 0 = 1).
A second-order reaction refers to one whose rate is dependent on the square of the concentration of a single reactant (e.g., in a homo-dimerization reaction, A + A → A2) or the combined first-order dependence on the concentrations of two different reactants (A + B → C). In this case: rate = [A]2 the reaction is zero order with respect to B because the concentration of B doesn't affect the rate of the reaction. The order with respect to A is 2 - it's a second order reaction with respect to A. The reaction is also second order overall (because 0 + 2 = 2).
Orders of reaction are always found by doing experiments.
Example 1.#
At high temperatures, propyl chloride produces HCl and propylene by the following reaction:
| Experiment | [CH3CH2CH2Cl]0 (M) | Initial Rate (M/s) |
| 1 | 0.020 | 1.2 × 10−8 |
| 2 | 0.025 | 1.8 × 10−8 |
| 3 | 0.040 | 2.4 × 10−8 |
| 4 | 0.080 | 4.8 × 10−8 |
Given: balanced chemical equation, initial concentrations of reactant, and initial rates of reaction
Asked for: reaction order and rate constant
Strategy:
A. Compare the data from two experiments to determine the effect on the reaction rate of changing the concentration of a species.
B. Compare the observed effect with behaviors characteristic of zeroth- and first-order reactions to determine the reaction order. Write the rate law for the reaction.
C. Use measured concentrations and rate data from any of the experiments to find the rate constant.
Solution:
The reaction order with respect to propyl chloride is determined by examining the effect of changes in the propyl chloride concentration on the reaction rate.
A. Comparing Experiments 2 and 3 shows that doubling the concentration doubles the reaction rate, so the reaction rate is proportional to [CH3CH2CH2Cl]. Similarly, comparing Experiments 1 and 4 shows that quadrupling the concentration quadruples the reaction rate, again indicating that the reaction rate is directly proportional to [CH3CH2CH2Cl].
B. This behavior is characteristic of a first-order reaction, for which the rate law is rate = k[CH3CH2CH2Cl].
C. We can calculate the rate constant (k) using any row in the table. Selecting Experiment 1 gives the following:
Example 2.#
At high temperatures, sulfur trioxide decomposes to sulfur dioxide and oxygen.
2SO3(g) → 2SO2(g) + O2(g)
Experimental data for the reaction at 300°C and four initial concentrations of SO3 are listed in the following table:
| Experiment | [SO3]0 (M) | Initial Rate (M/s) |
| 1 | 0.18 | 0.99 × 10−3 |
| 2 | 0.12 | 4.40 × 10−4 |
| 3 | 0.096 | 2.8 × 10−4 |
| 4 | 0.06 | 1.1 × 10−4 |
Determine the reaction order and the rate constant.
Given: balanced chemical equation, initial concentrations, and initial rates
Asked for: reaction order and rate constant
Strategy:
A. From the experiments, compare the changes in the initial reaction rates with the corresponding changes in the initial concentrations. Determine whether the changes are characteristic of zeroth-, first-, or second-order reactions.
B. Determine the appropriate rate law. Using this rate law and data from any experiment, solve for the rate constant (k).
Solution:
A. We can determine the reaction order with respect to sulfur trioxide by comparing the changes in SO3 concentrations with the corresponding reaction rates. Comparing Experiments 2 and 4, for example, shows that doubling the concentration quadruples the reaction rate [(4.40 × 10−4) ÷ (1.1 × 10−5) = 4.0], which means that the reaction rate is proportional to [SO3]2. Similarly, comparing Experiments 1 and 4 shows that tripling the concentration increases the reaction rate by a factor of 9, again indicating that the reaction rate is proportional to [SO3]2. This behavior is characteristic of a second-order reaction.
B. We have rate = k[SO3]2. We can calculate the rate constant (k) using data from any experiment in the table. Selecting Experiment 2, for example, gives the following:
Example 3.#
The following table lists kinetics data for the reaction of CO with O2 at 90°C:
2CO(g) + O2(g) →2CO2(g)
Determine the rate law for the reaction and calculate the rate constant.
| Experiment | [CO]0 (M) | [O2]0 (M) | Initial Rate (M/s) |
| 1 | 0.035 | 0.025 | 6.5 × 10−4 |
| 2 | 0.035 | 0.050 | 13 × 10−4 |
| 3 | 0.070 | 0.025 | 26.0 × 10−4 |
| 4 | 0.070 | 0.050 | 51.72 × 10−4 |
Given: balanced chemical equation, initial concentrations, and initial rates
Asked for: rate law and rate constant
Strategy:
A. Compare the changes in initial concentrations with the corresponding changes in rates of reaction to determine the reaction order for each species. Write the rate law for the reaction.
B. Using data from any experiment, substitute appropriate values into the rate law. Solve the rate equation for k.
Solution:
A. Comparing Experiments 1 and 2 shows that as [O2] is doubled at a constant value of [CO2], the reaction rate approximately doubles. Thus the reaction rate is proportional to [O2]1, so the reaction is first order in O2. Comparing Experiments 1 and 3 shows that the reaction rate essentially quadruples when [CO] is doubled and [O2] is held constant. That is, the reaction rate is proportional to [CO]2, which indicates that the reaction is second order in CO. Using these relationships, we can write the rate law for the reaction:rate=k[CO]2[O2]
B. The data in any row can be used to calculate the rate constant. Using Experiment 1, for example, gives
The overall reaction order (m + n) is 3, so this is a third-order reaction, a reaction whose rate is determined by three reactants. The units of the rate constant become more complex as the overall reaction order increases.
Сheck yourself:
Rates of reaction between NO and H2 at 800°C. Determine the reaction order and the rate constant.
Experiment NO H2 Initial Rate of Reaction
Number moles/L moles/L moles/L ∙ sec 1 0.001 0.004 0.002 2 0.002 0.004 0.008 3 0.003 0.004 0.018 4 0.004 0.001 0.008 5 0.004 0.002 0.016 6 0.004 0.003 0.024 Explain a solution strategy using key expressions.
24. Calculating an Equilibrium Constant from Equilibrium Concentrations and vice versa#
Notes:
When an equilibrium constant is calculated from equilibrium concentrations, molar concentrations or partial pressures are substituted into the equilibrium constant expression for the reaction. Equilibrium constants can be used to calculate the equilibrium concentrations of reactants and products by using the quantities or concentrations of the reactants, the stoichiometry of the balanced chemical equation for the reaction, and a tabular format to obtain the final concentrations of all species at equilibrium.
The reaction between gaseous carbon monoxide and water is used to obtain hydrogen:
Given: balanced equilibrium equation and composition of equilibrium mixture
Asked for: equilibrium constant
Strategy:
Write the equilibrium constant expression for the reaction. Then substitute the appropriate equilibrium concentrations into this equation to obtain K.
Solution:
Substituting the appropriate equilibrium concentrations into the equilibrium constant expression:
Answer: equilibrium constant is 0.0833
Example 2.#
A 2.00 mol sample of SO3 was placed in a 4.00 L reactor and heated to 140°C until the system reached equilibrium. The contents of the reactor were then analyzed and found to contain 0.56 mol of SO2. Calculate K at this temperature. The equation for the decomposition of SO3 to SO2 and O2 is as follows:
Given: balanced equilibrium equation, amount of reactant, volume, and amount of one product at equilibrium
Asked for: K
Strategy:
A. Write the equilibrium constant expression for the reaction. Construct a table showing the initial concentrations, the changes in concentrations, and the final concentrations (as initial concentrations plus changes in concentrations).
B. Calculate all possible initial concentrations from the data given and insert them in the table.
C. Use the coefficients in the balanced chemical equation to obtain the changes in concentration of all other substances in the reaction. Insert those concentration changes in the table.
D. Obtain the final concentrations by summing the columns. Calculate the equilibrium constant for the reaction.
Solution:
The first step in any such problem is to balance the chemical equation for the reaction (if it is not already balanced) and use it to derive the equilibrium constant expression. In this case, the equation is already balanced, and the equilibrium constant expression is as follows:
To obtain the concentrations of SO3, SO2, and O2 at equilibrium, we construct a table showing what is known and what needs to be calculated. We begin by writing the balanced chemical equation at the top of the table, followed by three lines corresponding to the initial concentrations, the changes in concentrations required to get from the initial to the final state, and the final concentrations.
| 2SO3(g) ⇄ 2SO2(g) + O2(g) | |||
| [SO3] | [SO2] | [O2] | |
| initial | |||
| change | |||
| final | |||
B. Initially, the system contains 2.00 mol of SO3 in a 4.00 L container. Thus [SO3]i = = 0.5 M. The initial concentrations of SO2 and O2 are 0 M because initially no products are present. Moreover, we are told that at equilibrium the system contains 0.56 mol of SO2 in a 4.00 L container, so [SO2]f = = 0.14 M. We insert these values into the following table:
| 2SO3(g) ⇄ 2SO2(g) + O2(g) | |||
| [SO3] | [SO2] | [O2] | |
| initial | 0.5 | 0 | 0 |
| change | |||
| final | 0.14 | ||
C. We use the stoichiometric relationships given in the balanced chemical equation to find the change in the concentration of SO2, the substance for which initial and final concentrations are known:
Δ[SO2] = [0.14 M (final) − 0.00 M (initial)] = +0.14 M
According to the coefficients in the balanced chemical equation, 1 mol of O2 are produced for every 2 mol of SO2, so the change in the O<sub,>2</sub,> concentration is as follows:
Δ [O2] = = 0.07M O2
Similarly, 2 mol of SO3 are consumed for every 1 mol of O2 produced, so the change in the SO3 concentration is as follows:
Δ[SO3] = 2 ∙ Δ[O2] = 0.14 M
We insert these values into our table:
| 2SO3(g) ⇄ 2SO2(g) + O2(g) | |||
| [SO3] | [SO2] | [O2] | |
| initial | 0.5 | 0 | 0 |
| change | -0.14 | +0.14 | +0.07 |
| final | 0.14 | ||
D. We sum the numbers in the [SO3] and [O2] columns to obtain the final concentrations of O2 and SO3:
[O2]f = 0.000 M + 0.07M = 0.07M
[SO3]f = 0.5M -0.14M = 0.36M
We can now complete the table:
| 2SO3(g) ⇄ 2SO2(g) + O2(g) | |||
| [SO3] | [SO2] | [O2] | |
| initial | 0.5 | 0 | 0 |
| change | -0.14 | +0.14 | +0.07 |
| final | 0.36 | 0.14 | 0.07 |
We can now calculate the equilibrium constant for the reaction:
Answer: Keq= 0.01
Example 3.#
Using the reaction, A (g) + B (g) ⇌C (g) + D (g) K = 0.20 at 400 K. If a gas mixture that initially contains 0.02 M A and 0.02 M B makes it possible to balance at 400 K, what are the final concentrations of all the substances present?
Given: balanced equilibrium equation, K, and initial concentrations
Asked for: final concentrations
Strategy:
A. Construct a table showing what is known and what needs to be calculated. Define x as the change in the concentration of one substance. Then use the reaction stoichiometry to express the changes in the concentrations of the other substances in terms of x. From the values in the table, calculate the final concentrations.
B. Write the equilibrium equation for the reaction. Substitute appropriate values from the table to obtain x.
C. Calculate the final concentrations of all species present. Check your answers by substituting these values into the equilibrium constant expression to obtain K.
Solution: A. The initial concentrations of the reactants are [A]i = [B]i = 0.02 M. Just as before, we will focus on the change in the concentrations of the various substances between the initial and final states. If we define the change in the concentration of C as x, then Δ[C] = +x. We can use the stoichiometry of the reaction to express the changes in the concentrations of the other substances in terms of x. For example, 1 mol of D is produced for every 1 mol of C, so the change in the D concentration can be expressed as Δ[D] = +x. Similarly, for every 1 mol of C produced, 1 mol each of A and B are consumed, so the change in the concentration of the reactants is Δ[A] = Δ[B] = −x. We enter the values in the following table and calculate the final concentrations.
| A(g) + B(g) ⇌C(g) + D(g) | ||||
| [A] | [B] | [C] | [D] | |
| initial | 0.02 | 0.02 | 0 | 0 |
| change | −x | −x | +x | +x |
| final | (0.02 − x) | (0.02 − x) | x | x |
B. We can now use the equilibrium equation and the given K to solve for x:
Taking the square root of the middle and right terms
C. The final concentrations of all species in the reaction mixture are as follows:
[A]final=[A]initial +Δ[A] = (0.02-0.00618) = 0.013782
[B]final=[B]initial + Δ[B] = (0.02-0.00618) = 0.013782
[C]final=[C]initial + Δ[C] = (0+0.00618) = 0.00618
[D]final=[D]initial + Δ[D] = (0+0.00618) = 0.00618
We can check our work by inserting the calculated values back into the equilibrium constant expression:
To two significant figures, this K is the same as the value given in the problem, so our answer is confirmed.
Answer:
[A]final = 0.013782
[B]final= 0.013782
[C]final= 0.00618
[D]final= 0.00618
Сheck yourself:
A 3.00 mol sample of CO was placed in a 2.00 L reactor and heated to 80°C until the system reached equilibrium. The contents of the reactor were then analyzed and found to contain 0.12 mol of CO2. Calculate K at this temperature. The equation for the synthesis of CO2 from CO and O2 is as follows: 2CO(g)+O2(g) ⇄ 2CO2(g)
Explain a solution strategy using key expressions.
25. Calculating ΔS from Standard Molar Entropy Values#
Notes:
Entropy increases with softer, less rigid solids, solids that contain larger atoms, and solids with complex molecular structures.
ΔS° for a reaction can be calculated from absolute entropy values using the same “products minus reactants” rule used to calculate ΔH°.
Example#
Use the data in Table 1 "Standard Molar Entropy Values of Selected Substances at 25°C" to calculate ΔS° for the reaction of liquid cyclohexane with O2(g) to give CO2(g) and H2O(g) at 298 K.
Table 1. Standard Molar Entropy Values of Selected Substances at 25°C
| Substance | S° [J/(mol·K)] |
| H2O | 188.8 |
| O2 | 205.2 |
| CO2 | 213.8 |
| CH3OH | 126.8 |
| CH3CH2OH | 160.7 |
| C6H12 (cyclohexane) | 204.4 |
Given: standard molar entropies, reactants, and products
Asked for: ΔS°
Strategy:
Write the balanced chemical equation for the reaction and identify the appropriate quantities in Table 1 "Standard Molar Entropy Values of Selected Substances at 25°C". Subtract the sum of the absolute entropies of the reactants from the sum of the absolute entropies of the products, each multiplied by their appropriate stoichiometric coefficients, to obtain ΔS° for the reaction.
Solution:
The balanced chemical equation for the complete combustion of cyclohexane (C6H12) is as follows:
We calculate ΔS° for the reaction using the “products minus reactants” rule, where m and n are the stoichiometric coefficients of each product and each reactant:
Answer: 364.4
Сheck yourself:
Use the data in Table 1 "Standard Molar Entropy Values of Selected Substances at 25°C" to calculate ΔS° for the reaction of liquid CH3OH with O2(g) to give CO2(g) and H2O(g) at 298 K.
Explain a solution strategy using key expressions.
26. Gibbs Free Energy and the Direction of Spontaneous Reactions#
Notes:
We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system in a new state function called Gibbs free energy (G).
The change in free energy (ΔG) is the difference between the heat released during a process and the heat released for the same process occurring in a reversible manner.
If a system is at equilibrium, ΔG = 0.
If the process is spontaneous, ΔG < 0.
If the process is not spontaneous as written but is spontaneous in the reverse direction, ΔG > 0.
At constant temperature and pressure, ΔG is equal to the maximum amount of work a system can perform on its surroundings while undergoing a spontaneous change. The standard free-energy change (ΔG°) is the change in free energy when one substance or a set of substances in their standard states is converted to one or more other substances, also in their standard states.
The standard free energy of formation (ΔG°f), is the change in free energy that occurs when 1 mol of a substance in its standard state is formed from the component elements in their standard states.
Tabulated values of standard free energies of formation are used to calculate ΔG° for a reaction.
Example 1.#
Calculate the standard free-energy change (ΔG°) at 25°C for the reaction H2(g)+O2(g)⇌H2O2(l). At 25°C, the standard enthalpy change (ΔH°) is −187.78 , and the absolute entropies of the products and reactants are S°(H2O2) = 109.6
, S°(O2) = 205.2
, and S°(H2) = 130.7
. Is the reaction spontaneous as written?
Given: balanced chemical equation, ΔH° and S° for reactants and products
Asked for: spontaneity of reaction as written
Strategy:
A. Calculate ΔS° from the absolute molar entropy values given.
B. Use Equation ΔG° = ΔH° − TΔS°, the calculated value of ΔS°, and other data given to calculate ΔG° for the reaction. Use the value of ΔG° to determine whether the reaction is spontaneous as written.
Solution:
A. To calculate ΔG° for the reaction, we need to know ΔH°, ΔS°, and T. We are given ΔH°, and we know that T = 298.15 K. We can calculate ΔS° from the absolute molar entropy values provided using the “products minus reactants” rule:
As we might expect for a reaction in which 2 mol of gas is converted to 1 mol of a much more ordered liquid, Δ S° is very negative for this reaction.
B. Substituting the appropriate quantities into Equation ΔG° = ΔH° − TΔS°,
Сheck yourself:
The hydrogenation of ethene gas at 298K shows a decrease in disorder (ΔS°= -120.7 J/(mol∙K)) during an exothermic reaction (ΔH° = -136.9 kJ/mol). Determine whether the reaction is spontaneous or nonspontaneous by calculating ΔG°. C2H4(g) + H2(g) → C2H6(g)
Explain a solution strategy using key expressions.