💿 The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
note
This is an authentic video. Do not try to understand every word you hear.
Watch and listen for general and specific information by completing the following tasks.
Before you watch:#
Exercise 1. Study the problem. Can you solve it?#
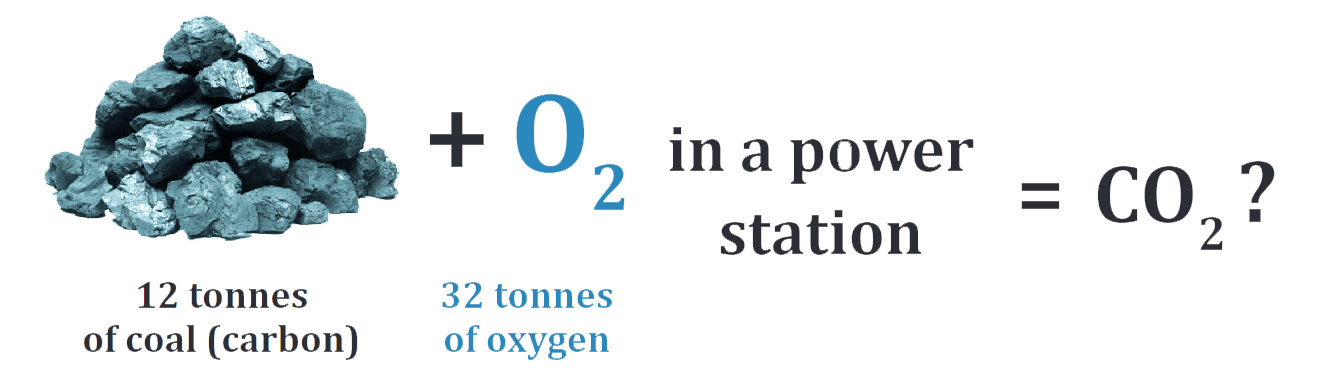
Video#
While you watch:#
Exercise 2. Watch the video (till 0:50) and check your answer to the problem question from Exercise 1. Then, complete the gaps in the sentence.#
Exercise 3. Watch the video (0:51-1:07) and study the next task given. Then, watch the video further (till the end) and complete the flowchart explaining FOUR STEPS to make this calculation:#
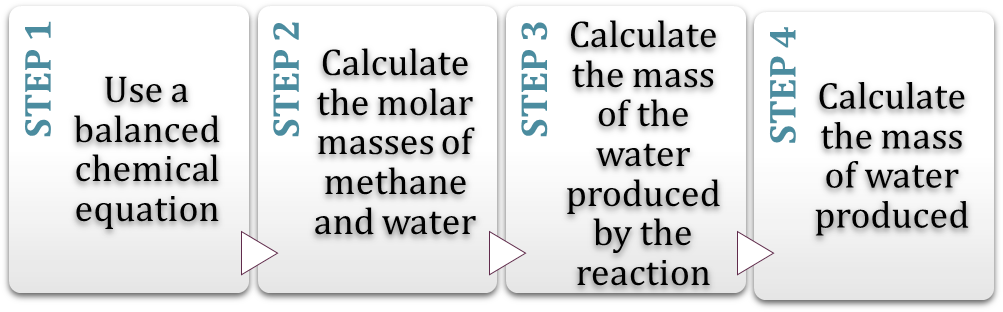
After you watch:#
Exercise 4: Solve the following problems:#
Define the mass of CO2 which is produced from complete combustion of 36 liters of propane.
How many grams of sulfuric acid needed to produce 5,6 liters (n.c.) of hydrogen while reacting with zink?