Bloom Taxonomy
- Task
- Practice Exercises
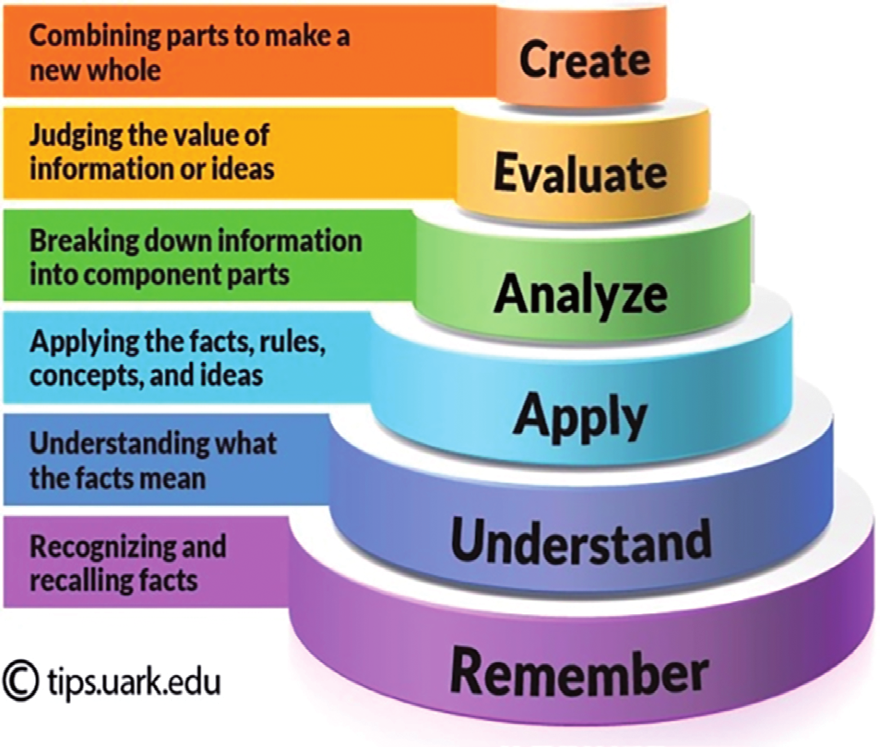
| create | A student carried out a laboratory experiment to determine the enthalpy change when a sample of ethanol was burned. The heat produced was used to warm some water in a copper calorimeter. The student found that the temperature of 75.0 g of water increased by 5.50 °C when 2.40 × 10–3 mol of pure ethanol was burned in air. Deduce two reasons why the student’s value for the standard enthalpy of combustion of ethanol is different from a Data Book value of –1279 kJ mol–1. |
| evaluate | During intense exercise, your body cannot provide enough oxygen to allow the complete combustion of glucose to carbon dioxide. Under these conditions, an alternative means of obtaining energy from glucose is used in which glucose (C6H12O6) is converted to lactic acid (C3H5O3H). The equation for this reaction is as follows: C6H12O6 → 2 C3H5O3H Calculate the energy yield for this reaction per mole of glucose. How does this energy yield compare with that obtained per mole of glucose for the combustion reaction? Muscles become sore after intense exercise. Propose a chemical explanation for this. |
| analyse | What is the difference between ΔHof and ΔHf? |
| apply | Draw an enthalpy profile diagram for photosynthesis: 6CO2 + 6H2O → C6H12O6 + 6O2 ΔH = +2802 kJmol-1. Label the reactants and products, enthalpy change and activation energy. |
| understand | Describe the energy changes which take place in an endothermic chemical reaction. |
| remember | What is meant by the term ‘exothermic reaction’? |