Bloom Taxonomy
- Task
- Practice Exercises
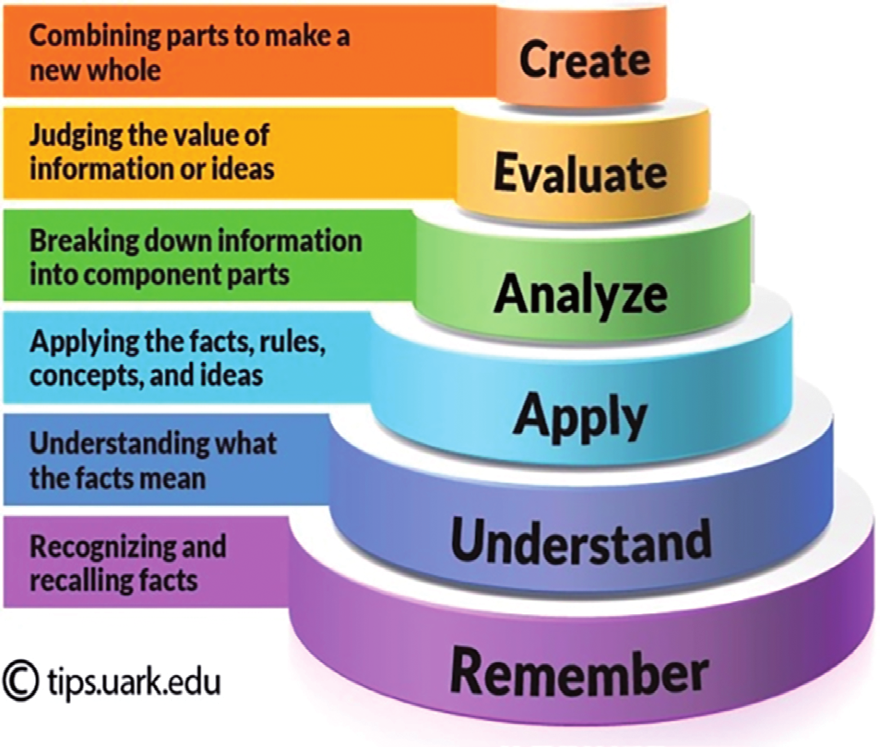
| create | Explain, why cracking is economically and environmentally important. |
| evaluate | Using a free radical substitution mechanism, suggest steps to form a 1,1-dichloroethane molecule. |
| analyse | The boiling points of some straight-chain alkanes: C4H10 (-0.5°C), C5H12 (36.3) C6H14 (68.7). Explain the trend in these boiling points. |
| apply | Halothane is used as an anesthetic and has the following formula: С2HF3BrCl. Calculate percentage by mass of fluorine in halothane. |
| understand | Write an equation to show how one molecule of C14H30 is cracked to form one molecule of C8H18 and one molecule of another hydrocarbon. |
| remember | State what is meant by the term saturated, as applied to hydrocarbons. |