Bloom Taxonomy
- Task
- Practice Exercises
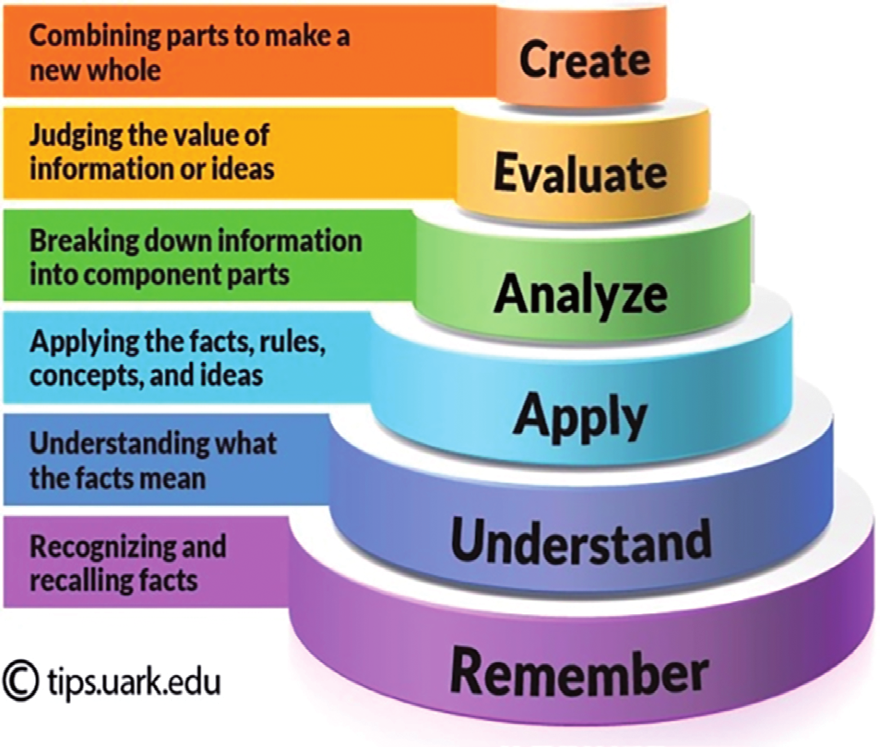
| create | Ozone, O3, is produced in the stratosphere by the chemical reaction 3O2(g)→2O3(g) If at a given instant, molecular oxygen, O2, is reacting at a rate of 2.17×10−5M/s , at what rate is ozone being produced? |
| evaluate | The oxidation of ammonia produces nitrogen and water via the reaction 4NH3 + 3O2 → 2N2 + 6H2O. If the rate of formation of N2 is 2.0 |
| analyse | Of two highly exothermic reactions with different values of Ea, which would need to be monitored more carefully: the one with the smaller value or the one with the higher value? Why? |
| apply | Atmospheric chemistry in the region below the clouds of Venus appears to be dominated by reactions of sulfur and carbon-containing compounds. Included in representative elementary reactions are the following SO2 + CO → SO + CO2 SO + CO → S + CO2 SO + SO2 → S + SO3 For each elementary reaction, write an expression for the net rate of reaction in terms of the concentrations of reactants and products. |
| understand | Explain why not all collisions lead to a chemical reaction. |
| remember | What information can you get from the reaction order? What correlation does the reaction order have with the stoichiometry of the overall equation? |