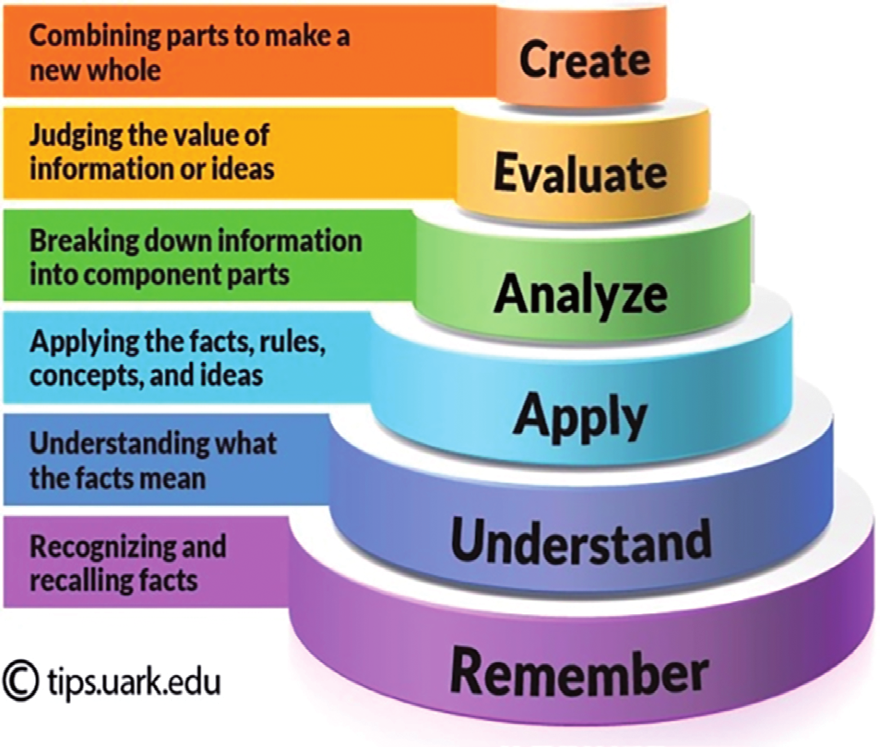
| create | Suggest an algorithm for balancing redox reactions NO2– + MnO4– → NO3– + Mn+2 (in acid solution) using the half-reaction method. |
| evaluate | Describe the trend in the oxidation number for main-group elements as you move left-to-right across a row in the periodic table. |
| analyse | Compare the oxidation state of hydrogen in HCl with its oxidation state in NaH. Why are they different? |
| apply | Determine the oxidation number of the elements in each of the following compounds: H2CO3, NO2–, Fe3O4 |
| understand | Would you use an oxidizing agent or reducing agent for the following reactions to occur: ClO3– → ClO2–; Mn2+ → MnO2; SO42- → S2-? |
| remember | |