Bloom Taxonomy
- Task
- Practice Exercises
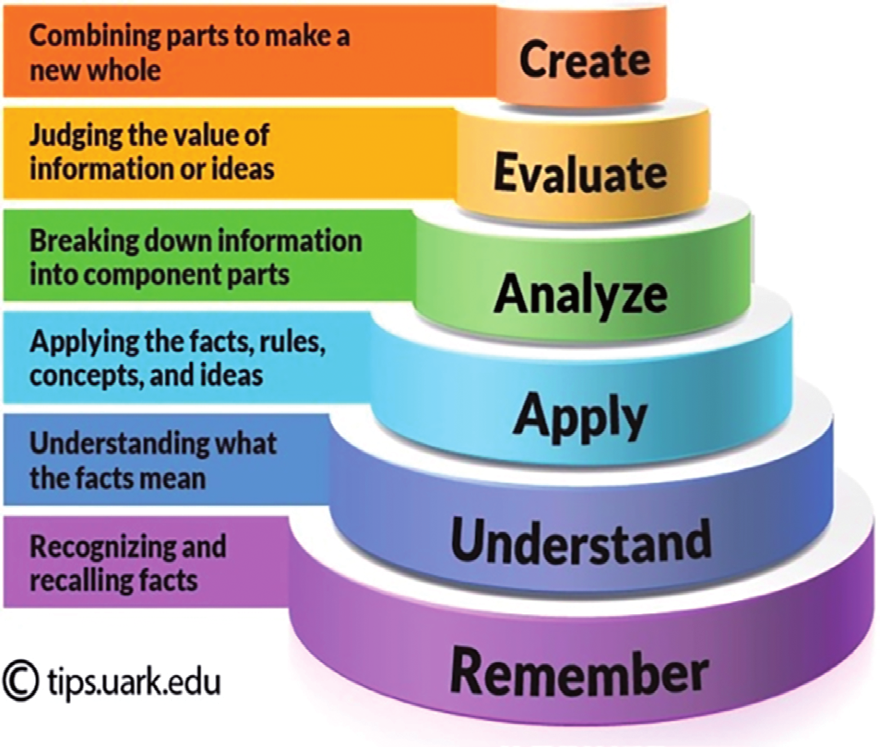
| create | There is this equilibrium CH3CH2COOH + CH3CH2OH ⇌ CH3CH2COOCH2CH3 + H2O ∆Hο = –22 kJ mol–1 . Predict the effect of an increase in temperature on the amount, in moles, of ester at equilibrium |
| evaluate | The decomposition of ammonium carbamate to NH3 and CO2 at 30°C is written as NH4CO2NH2(s) ⇌ 2NH3(g) + CO2(g). If the partial pressure of NH3 at equilibrium is 0.124 atm, what is the equilibrium partial pressure of CO2? What is the total gas pressure of the system? |
| analyse | Classify each equilibrium system as either homogeneous or heterogeneous: a) 2HF(g) ⇌ H2(g)+F2(g) b) C(s)+2H2(g)⇌CH4(g) c) H2C=CH2(g)+H2(g)⇌C2H2(g) d) 2Mg(s)+O2(g)⇌2MgO(s) |
| apply | For the equilibrium system 3O2(g) ⇌ 2O3(g), write the equilibrium constant expression Kp. |
| understand | If an equilibrium reaction is endothermic in the forward direction, what is the expected change in the concentration of each component of the system if the temperature of the reaction is increased? If the temperature is decreased? |
| remember | State the three features of a dynamic equilibrium. |