📝 Electron Shell of an atom
- Task
- Practice Exercises
🎧️ Listen to the recording and mind pronunciation of words.#
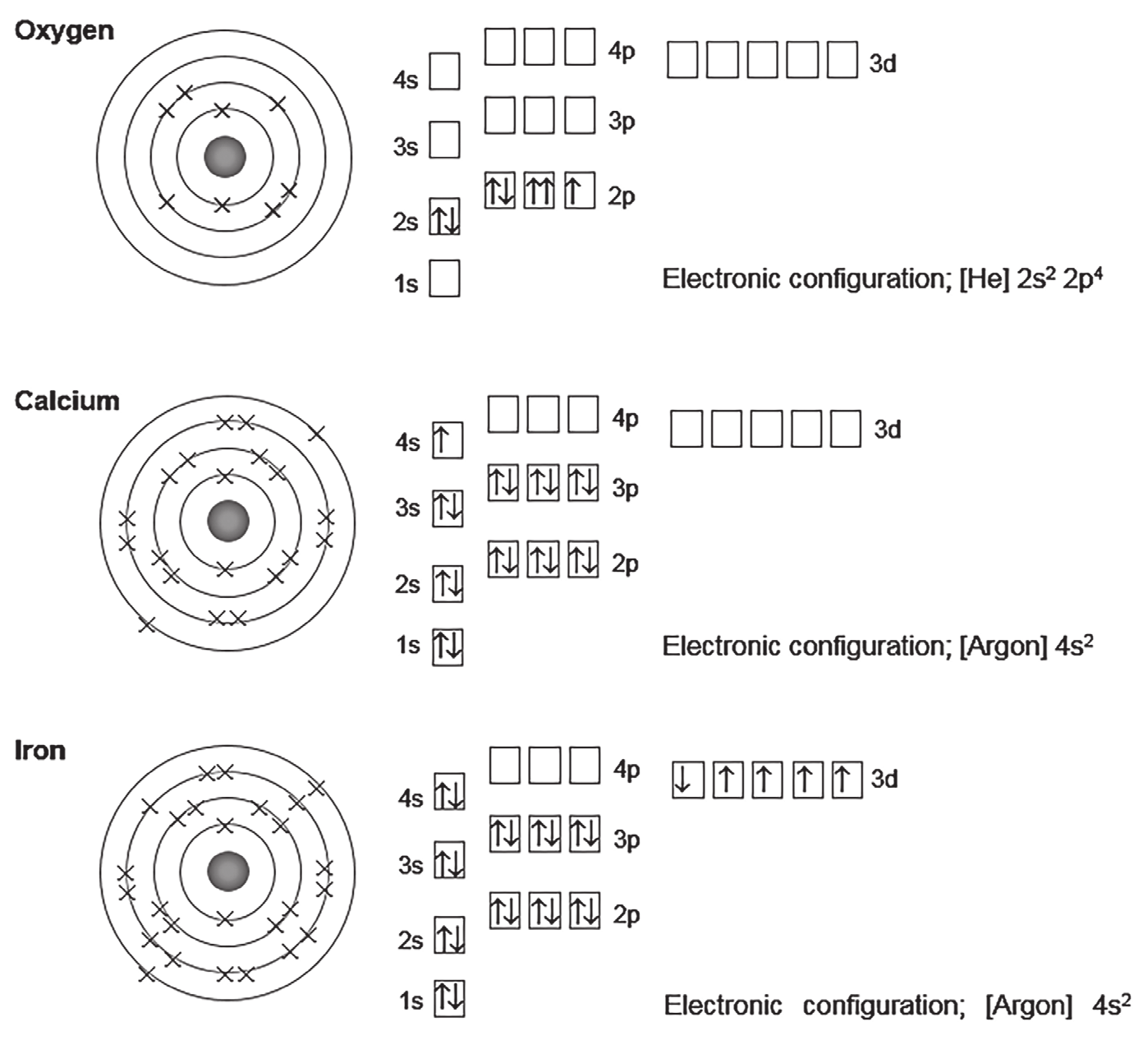
The arrangement of electrons in an atom is called its electronic structure or electron configuration. Electrons are arranged outside the nucleus in energy levels or quantum shells. These principal energy levels or principal quantum shells (symbol n) are numbered according to how far they are from the nucleus.Each principal quantum shell can hold a maximum number of electrons:
shell 1 – up to 2 electrons
shell 2 – up to 8 electrons
shell 3 – up to 18 electrons
shell 4 – up to 32 electrons.
The principal quantum shells, apart from the first, are split into subshells.Each subshell contains one or more atomic orbitals.An atomic orbital is a region of space around the nucleus of an atom that can be occupied by one or two electrons.The number of orbitals in each subshell must be:
s – one orbital
p – three orbitals
d – five orbitals
An s orbital has a spherical shape. Electrons in the 2s orbital have more energy than electrons in the 1s orbital. The shape of p orbitals is like an hourglass with two ‘lobes’. A d orbital is like a modified p orbital with a ring round the middle.
When two electrons are present in an orbital they spin in opposite directions and are said to be paired. The electronic configuration of atoms is found by adding electrons to each orbital starting from those in the lowest energy level. When electrons are added to orbitals in the same subshell they go into separate orbitals if possible. Electrons pair up when this is not possible.
The 1st ionisation energy of an element is the energy needed to remove one electron from each atom in one mole of atoms of the element in the gaseous state to form a mole of gaseous 1+ ions.
As the atomic number increases, the positive nuclear charge increases. The bigger the positive charge, the greater the attractive force between the nucleus and the electrons. So,more energy is needed to overcome these attractive forces if an electron is to be removed. Ionisation energy increases as the proton number increases. The further the outer electron shell is from the nucleus, the lower the ionisation energy. Full inner shells of electrons prevent the full nuclear charge being felt by the outer electrons. This is called shielding. The ionisation energy is lower as the number of full electron shells between the outer electrons and the nucleus increases.
The magnitude of the ionisation energy depends on these four factors:
– the distance of the electron from the nucleus
– the number of positive charges in the nucleus
– the degree of shielding of outer electrons by inner electron shells
– spin-pair repulsion.
2. Find the errors and write correctly#
Aufbau’s principle states that “electrons fill orbitals starting with the lowest energy orbital first”
Hund’s rule states that “when filling a set of orbitals of identical energy, electrons are added with parallel spins to different orbitals rather than pairing two electrons in the same orbital”
3. Find the errors and write them correctly. Trends in ionization energies:#
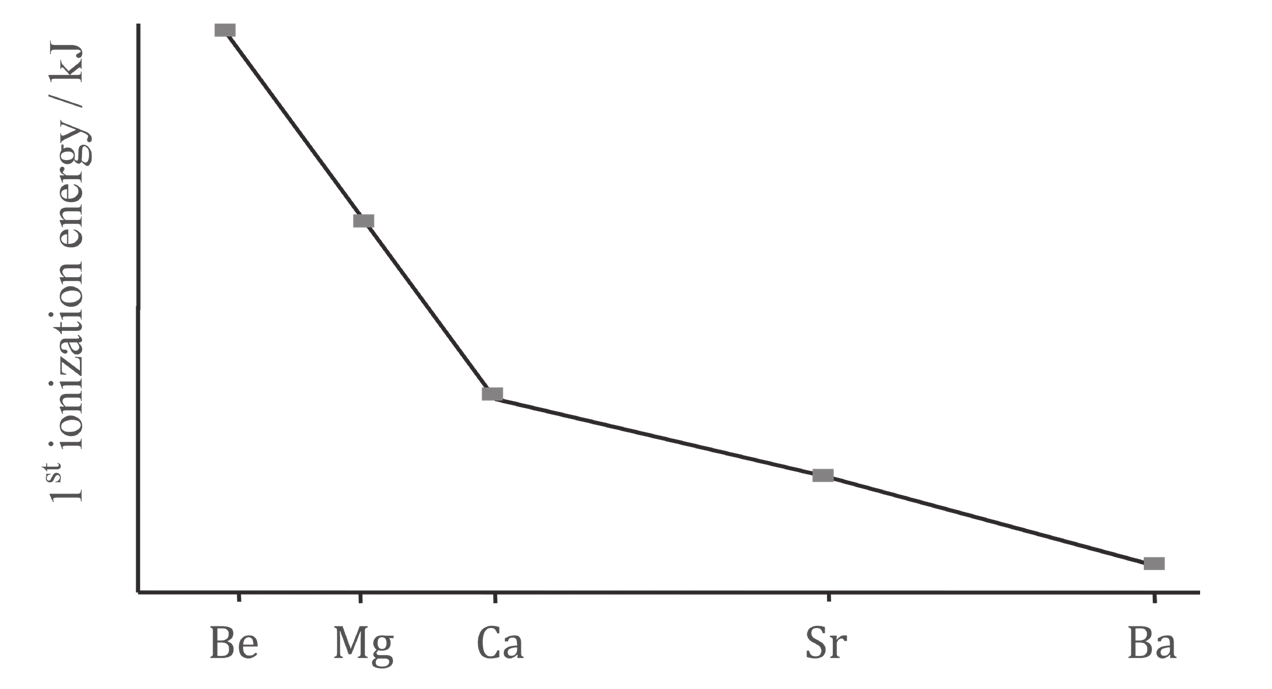
As you go up the group there is a general decrease in the first ionization energy. This is because:
- the outer electron is in a successively higher energy orbital and is further away from the positive pull of the nucleus. Hence more energy is needed to remove it.
- the number of electrons shielding the outer shell electron from the nuclear charge decreases (decreased shielding)
4. Find the errors and write correctly. Trends in ionization energies:#
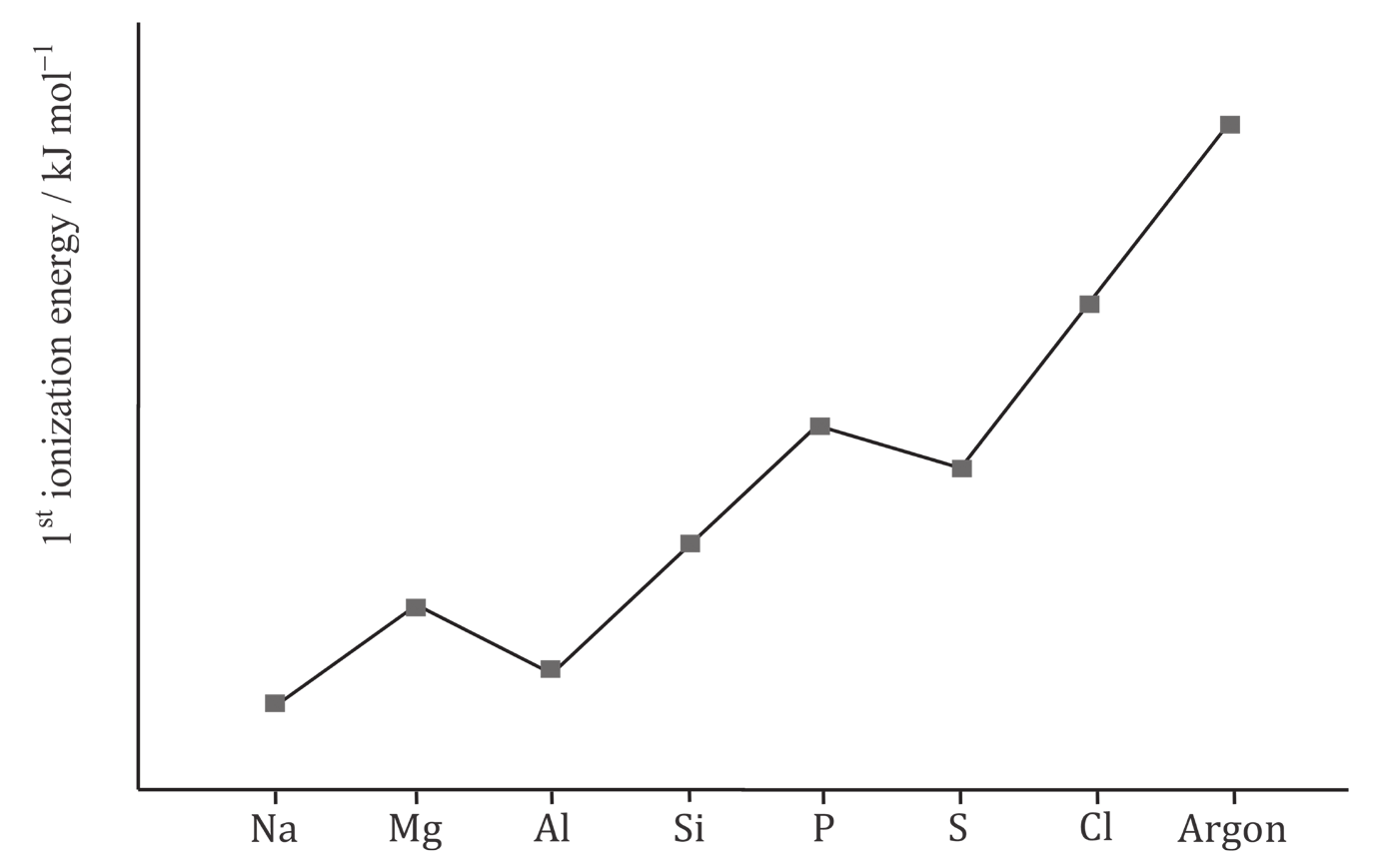
As you move across the group there is a general decrease in the first ionization energy. This is because there is a decrease in the nuclear charge as we go across the group and the electrons are being removed from the different principal energy level / the electrons are approximately the same distance from the nucleus.
The drop from Mg-Al is because the outer electron in Al is in a 2p orbital whereas the outer electron in Mg is in a 3s orbital. The 2p orbital is slightly higher in energy and so the electron is removed more easily.
The drop from P to S is a result of mutual attraction. In P, the 3 electrons in the 3p orbitals are in singly occupied orbitals. In S there are 4 electrons in the 3p orbitals meaning that one of the 3p orbitals must contain a pair of electrons. These attractions each other slightly meaning that it is easier to remove this electron than would be expected.
The drop from P to S is evidence of Pauli principle.