📝 Mass Spectrometry
- Task
- Practice Exercises
🎧️ Listen to the recording and mind pronunciation of words.#
The mass spectrometer is an instrument used for measuring the masses of atoms and molecules. It can also be used to measure the relative abundance of different isotopes and to predict the structure of more complex molecules.
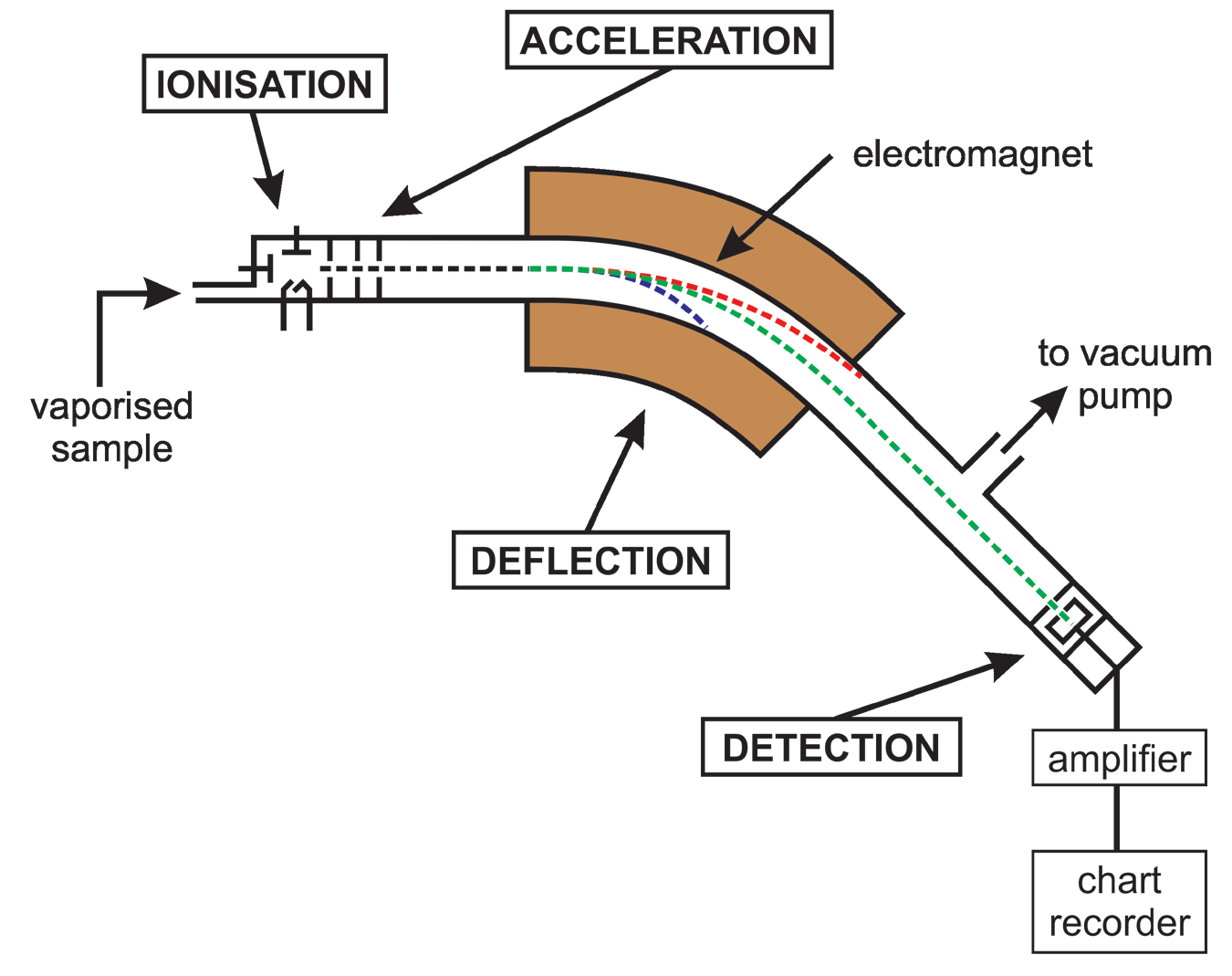
- The workings of the mass spectrometer can be summarized in five stages:
- Gaseous material released into ionization chamber.
- Particles bombarded with electrons and ionized, mostly to +1 ions (ionization).
A metal wire is heated until it starts emitting high energy electrons. These electrons hit the particles, knocking more electrons off. Most of the particles are ionized to +1 ions.- Particles bombarded with electrons and ionized, mostly to +1 ions (ionization).
- Ions accelerated to uniform speed by electric field (acceleration)
The positive ions are attracted to the negative plate and accelerate towards it.- Ions accelerated to uniform speed by electric field (acceleration)
- Ions deflected by magnetic field; deflection depends on m/e ratio (deflection).The heavier the particle, the less the deflection.
- Electric current measured as ions land on plate (detection)The greater the abundance of the isotope, the larger the current
In most cases, however, the charge is +1, so the deflection depends essentially on the relative mass of the species in the mass spectrometer. If the spectrometer is calibrated, the masses of all the species can be directly measured.
The greater the number of particles landing at a single point on the detector, the greater the electric current and the larger the peak. Thus the relative abundance of different isotopes can be measured.
Since the position at which an ion appears on the detector depends on its mass, different isotopes appear at different points on the detector. The magnitude of the peak gives the relative abundance of the isotope.
Thus the relative atomic mass of the element can be calculated from its mass spectrum.
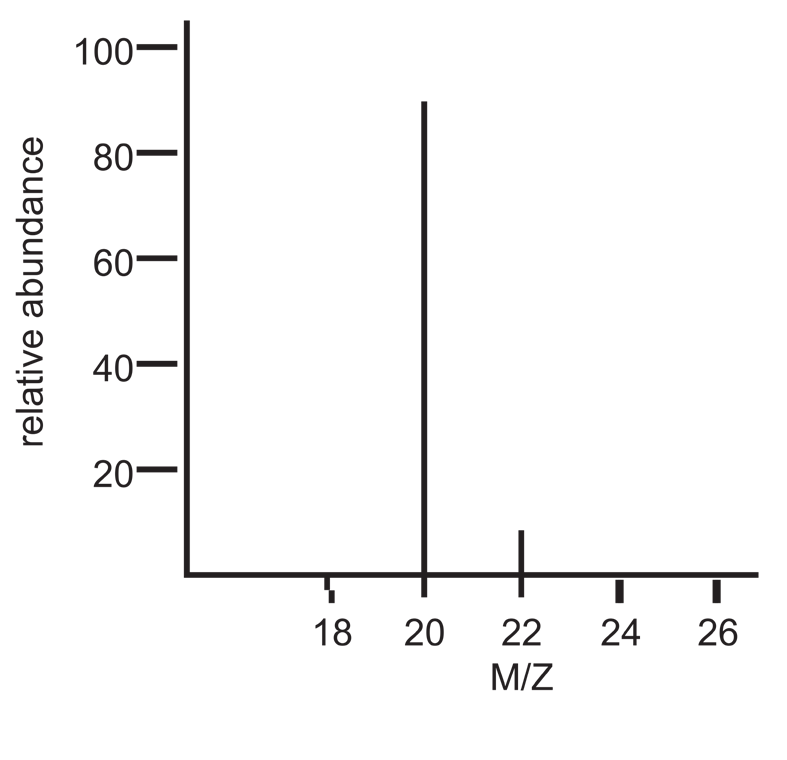
An example of a simple mass spectrum is shown below:
Mass spectrum of Ne
The peak at 20 is 20Ne+, and the peak at 22 is 22Ne+